Netarsudil-associated epithelial keratopathy
- PMID: 32637736
- PMCID: PMC7330490
- DOI: 10.1016/j.ajoc.2020.100800
Netarsudil-associated epithelial keratopathy
Abstract
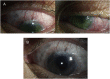
Purpose: To report 2 cases with a novel finding of bullous epithelial keratopathy associated with netarsudil use.
Observations: A 72-year-old man with history of primary open angle glaucoma was started on netarsudil daily in both eyes for uncontrolled intraocular pressures despite treatment with latanoprost, brimonidine, and dorzolamide-timolol. One month later he presented with bilateral conjunctival hyperemia, predominantly inferior corneal epithelial bullae, and keratic precipitates without hypopyon. Netarsudil was discontinued, and the patient was started on topical steroids. One week later, the hyperemia and corneal edema had resolved while many small keratic precipitates persisted.A 29-year-old man with history of rubella-associated glaucoma and chronic postoperative inflammation on prednisolone was started on netarsudil in his left eye only for elevated intraocular pressures despite latanoprost, brimonidine, and dorzolamide-timolol. Two months later, he complained of eye pain and decreased vision since starting netarsudil. Examination revealed mild hyperemia and inferior corneal epithelial bullae without keratic precipitates. Netarsudil was discontinued, and two weeks later, conjunctival injection resolved and cornea cleared.
Conclusions and importance: Netarsudil ophthalmic solution 0.02% (Rhopressa) is a rho-kinase inhibitor recently approved for lowering intraocular pressure in open-angle glaucoma or ocular hypertension. As netarsudil continues to be increasingly used, physicians and patients need to be aware of this new possible adverse effect.
Keywords: Drug reaction; Glaucoma; Keratopathy; Netarsudil; Rho-kinase inhibitors.
© 2020 Published by Elsevier Inc.
Conflict of interest statement
The following authors have no financial disclosures: MSR, VMA, AYL, PSS.
Figures

References
-
- ROCKET-1 and ROCKET-2 Study Groups Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2) Am J Ophthalmol. 2018 Feb;186:116–127. - PubMed
-
- ROCKET-4 Study Group Once-daily netarsudil versus twice-daily timolol in patients with elevated intraocular pressure: the randomized phase 3 ROCKET-4 study. Am J Ophthalmol. 2019 Aug;204:97–104. - PubMed
-
- Use of topical rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea. 2019 May;38(5):529–534. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

