Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing
- PMID: 32637741
- PMCID: PMC7317234
- DOI: 10.1016/j.bioactmat.2020.05.008
Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing
Abstract
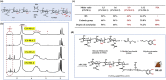
Uncontrolled bleeding and infection can cause significant increases in mortalities. Hydrogel sealants have attracted extensive attention for their ability to control bleeding. However, because interfacial water is a formidable barrier to strong surface bonding, a challenge remains in finding a product that offers robust tissue adhesion combined with anti-infection properties. Inspired by the strong adhesive mechanism of biofilm and mussels, we report a novel dual bionic adhesive hydrogel (DBAH) based on chitosan grafted with methacrylate (CS-MA), dopamine (DA), and N-hydroxymethyl acrylamide (NMA) via a facile radical polymerization process. CS-MA and DA were simultaneously included in the adhesive polymer for imitating the two key adhesive components: polysaccharide intercellular adhesin (PIA) of staphylococci biofilm and 3,4-dihydroxy-l-phenylalanine (Dopa) of mussel foot protein, respectively. DBAH presented strong adhesion at 34 kPa even upon three cycles of full immersion in water and was able to withstand up to 168 mm Hg blood pressure, which is significantly higher than the 60-160 mm Hg measured in most clinical settings. Most importantly, these hydrogels presented outstanding hemostatic capability under wet and dynamic in vivo movements while displaying excellent antibacterial properties and biocompatibility. Therefore, DBAH represents a promising class of biomaterials for high-efficiency hemostasis and wound healing.
Keywords: Adhesive; Antibacterial; Biofilm; Dual-biomimetic; Hemostasis; Hydrogel; Mussel.
© 2020 Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
There are no conflict of interest for our manuscript.
Figures









References
LinkOut - more resources
Full Text Sources

