Density Functional Theory Study on the Mechanism of Iridium-Catalyzed Benzylamine ortho C-H Alkenylation with Ethyl Acrylate
- PMID: 32637819
- PMCID: PMC7331057
- DOI: 10.1021/acsomega.0c01587
Density Functional Theory Study on the Mechanism of Iridium-Catalyzed Benzylamine ortho C-H Alkenylation with Ethyl Acrylate
Abstract
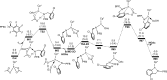
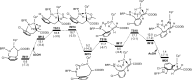
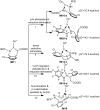
Iridium-catalyzed oxidative o-alkenylation of benzylamines with acrylates was enabled by the directing group pentafluorobenzoyl (PFB). Density functional theory calculations were performed to explore the detailed reaction mechanism. The calculated results reveal that N-deprotonation prior to C-H activation is favored over direct C-H activation. Moreover, C-H activation is reversible and not the rate-determining step, which has been supported by the experimental observation. The regio- and stereoselectivity of ethyl acrylate insertion are controlled by the steric effect and the carbon atom with a larger orbital coefficient of the π* antibonding orbital in the nucleophilic attack, respectively. The migratory insertion of ethyl acrylate is computationally found to be rate-determining for the whole catalytic cycle. Finally, the seven-membered ring intermediate IM11 undergoes a sequential N-protonation and β-H elimination with the assistance of AcOH, rather than β-H elimination and reductive elimination proposed experimentally, to afford the o-alkenylated product. IM11 is unable to directly cyclize through C-N reductive elimination because both sp3-hybridized N and C atoms are unfavorable for N-C reductive elimination. The origin of the directing group PFB preventing the product and intermediates undergoing aza-Michael addition has been explained.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
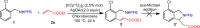




References
-
- Madia V. N.; Messore A.; Pescatori L.; Saccoliti F.; Tudino V.; De Leo A.; Bortolami M.; Scipione L.; Costi R.; Rivara S.; Scalvini L.; Mor M.; Ferrara F. F.; Pavoni E.; Roscilli G.; Cassinelli G.; Milazzo F. M.; Battistuzzi G.; Di Santo R.; Giannini G. Novel benzazole derivatives endowed with potent antiheparanase activity. J. Med. Chem. 2018, 61, 6918–6936. 10.1021/acs.jmedchem.8b00908. - DOI - PubMed
- Abonia R.; Garay A.; Castillo J.; Insuasty B.; Quiroga J.; Nogueras M.; Cobo J.; Butassi E.; Zacchino S. Design of two alternative routes for the synthesis of naftifine and analogues as potential antifungal agents. Molecules 2018, 23, 520.10.3390/molecules23030520. - DOI - PMC - PubMed
- Hirashima S.-i.; Narushima T.; Kawada M.; Nakashima K.; Hanai K.; Koseki Y.; Miura T. Asymmetric conjugate additions of carbonyl compounds to nitroalkenes under solvent-free conditions using fluorous diaminomethylenemalononitrile organocatalyst. Chem. Pharm. Bull. 2017, 65, 1185–1190. 10.1248/cpb.c17-00596. - DOI - PubMed
- Casimiro L.; Groppi J.; Baroncini M.; La Rosa M.; Credi A.; Silvi S. Photochemical investigation of cyanoazobenzene derivatives as components of artificial supramolecular pumps. Photochem. Photobiol. Sci. 2018, 17, 734–740. 10.1039/c8pp00062j. - DOI - PubMed
-
- Yang S.; Cheng R.; Zhao T.; Luo A.; Lan J.; You J. Rhodium-catalyzed C–H/C–H cross coupling of benzylthioethers or benzylamines with thiophenes enabled by flexible directing groups. Org. Lett. 2019, 21, 5086–5090. 10.1021/acs.orglett.9b01679. - DOI - PubMed
- Purkait A.; Jana C. K. N-aminations of benzylamines and alicyclic amines with nitrosoarenes to hydrazones and hydrazides. Synthesis 2019, 51, 2687–2696. 10.1055/s-0037-1610701. - DOI
- Liu Y.; Afanasenko A.; Elangovan S.; Sun Z.; Barta K. Primary benzylamines by efficient N-Alkylation of benzyl alcohols using commercial Ni catalysts and easy-to-handle ammonia sources. ACS Sustainable Chem. Eng. 2019, 7, 11267–11274. 10.1021/acssuschemeng.9b00619. - DOI - PMC - PubMed
-
- Mulet C.; Escolano M.; Llopis S.; Sanz S.; Ramírez de Arellano C.; Sánchez-Roselló M.; Fustero S.; del Pozo C. Dual role of vinyl sulfonamides as N-nucleophiles and Michael acceptors in the enantioselective synthesis of bicyclic delta-sultams. Adv. Synth. Catal. 2018, 360, 2885–2893. 10.1002/adsc.201800548. - DOI
- Hu Y.; Xie Y.; Shen Z.; Huang H. Palladium-catalyzed ring-forming aminoalkenylation of alkenes with aldehydes initiated by intramolecular aminopalladation. Angew. Chem., Int. Ed. 2017, 56, 2473–2477. 10.1002/anie.201611853. - DOI - PubMed
- Hu Y.; Shen Z.; Huang H. Palladium-catalyzed intramolecular hydroaminocarbonylation to lactams: additive-free protocol initiated by palladium hydride. ACS Catal. 2016, 6, 6785–6789. 10.1021/acscatal.6b01939. - DOI
- Kapoor M.; Chand-Thakuri P.; Young M. C. Carbon dioxide-mediated C(sp2)–H arylation of primary and secondary benzylamines. J. Am. Chem. Soc. 2019, 141, 7980–7989. 10.1021/jacs.9b03375. - DOI - PubMed
- Ogiwara Y.; Tamura M.; Kochi T.; Matsuura Y.; Chatani N.; Kakiuchi F. Ruthinium-catalyzed ortho-selective C-H alkenylation of aromatic compounds with alkenyl esters and ethers. Organometallics 2014, 33, 402–420. 10.1021/om401204h. - DOI
- Lin H.; Pan X.; Barsamian A. L.; Kamenecka T. M.; Bannister T. D. Native directed site-selective δ-C(sp3)-H and δ-C(sp2)-H arylation of primary amines. ACS Catal. 2019, 9, 4887–4891. 10.1021/acscatal.8b04927. - DOI
- Pramanick P. K.; Zhou Z.; Hou Z.-L.; Yao B. Free amino group-directed γ-C(sp3)–H arylation of α-amino esters with diaryliodonium triflates by palladium catalysis. J. Org. Chem. 2019, 84, 5684–5694. 10.1021/acs.joc.9b00605. - DOI - PubMed
-
- Wang S.-M.; Moku B.; Leng J.; Qin H.-L. Rh-catalyzed carboxylates directed C–H activation for the synthesis of ortho-carboxylic 2-arylethenesulfonyl fluorides: access to unique electrophiles for SuFEx click chemistry. Eur. J. Org. Chem. 2018, 4407–4410. 10.1002/ejoc.201800762. - DOI
- Lv H.; Shi J.; Huang J.; Zhang C.; Yi W. Rhodium(III)-catalyzed and MeOH-involved regioselective monoalkenylation of N-aryureas with acrylates. Org. Biomol. Chem. 2017, 15, 7088–7092. 10.1039/c7ob01627a. - DOI - PubMed
- Patureau F. W.; Glorius F. Rh catalyzed olefination and vinylation of unactivated acetanilides. J. Am. Chem. Soc. 2010, 132, 9982–9983. 10.1021/ja103834b. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

