Myelin Basic Protein Phospholipid Complexation Likely Competes with Deimination in Experimental Autoimmune Encephalomyelitis Mouse Model
- PMID: 32637820
- PMCID: PMC7331039
- DOI: 10.1021/acsomega.0c01590
Myelin Basic Protein Phospholipid Complexation Likely Competes with Deimination in Experimental Autoimmune Encephalomyelitis Mouse Model
Abstract
Multiple sclerosis has complex pathogenesis encompassing a variety of components (immunologic, genetic, and environmental). The autoimmunogenicity against the host's myelin basic protein is a major contributor. An increase in myelin basic protein deimination (a post-translational modification) and a change in phospholipid composition have been associated with multiple sclerosis. The interaction of myelin basic protein with phospholipids in the myelin membrane is an important contributor to the stability and maintenance of proper myelin sheath function. The study of this aspect of multiple sclerosis is an area that has yet to be fully explored and that the present study seeks to understand. Several biochemical methods, a capillary electrophoresis coupled system and mass spectrometry, were used in this study. These methods identified four specific phospholipids complexing with myelin basic protein. We show that lysophosphatidylcholine 18:1 provides a robust competitive effect against hyper-deimination. Our data suggest that lysophosphatidylcholine 18:1 has a different biochemical behavior when compared to other phospholipids and lysophosphatidylcholines 14:0, 16:0, and 18:0.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

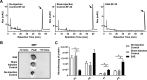

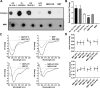

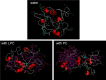
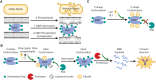
References
-
- Lassmann H. What drives disease in multiple sclerosis: Inflammation or neurodegeneration?. Clin. Exp. Neuroimmunol. 2010, 1, 2–11. 10.1111/j.1759-1961.2009.00003.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

