Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19
- PMID: 32638436
- PMCID: PMC7361465
- DOI: 10.1111/apt.15916
Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19
Abstract
Background: The incidence of elevated liver chemistries and the presence of pre-existing chronic liver disease (CLD) have been variably reported in COVID-19.
Aims: To assess the prevalence of CLD, the incidence of elevated liver chemistries and the outcomes of patients with and without underlying CLD/elevated liver chemistries in COVID-19.
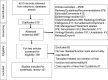
Methods: A comprehensive search of electronic databases from 1 December 2019 to 24 April 2020 was done. We included studies reporting underlying CLD or elevated liver chemistries and patient outcomes in COVID-19.
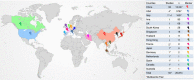
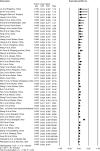
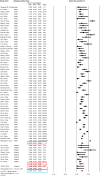
Results: 107 articles (n = 20 874 patients) were included for the systematic review. The pooled prevalence of underlying CLD was 3.6% (95% CI, 2.5-5.1) among the 15 407 COVID-19 patients. The pooled incidence of elevated liver chemistries in COVID-19 was 23.1% (19.3-27.3) at initial presentation. Additionally, 24.4% (13.5-40) developed elevated liver chemistries during the illness. The pooled incidence of drug-induced liver injury was 25.4% (14.2-41.4). The pooled prevalence of CLD among 1587 severely infected patients was 3.9% (3%-5.2%). The odds of developing severe COVID-19 in CLD patients was 0.81 (0.31-2.09; P = 0.67) compared to non-CLD patients. COVID-19 patients with elevated liver chemistries had increased risk of mortality (OR-3.46 [2.42-4.95, P < 0.001]) and severe disease (OR-2.87 [95% CI, 2.29-3.6, P < 0.001]) compared to patients without elevated liver chemistries.
Conclusions: Elevated liver chemistries are common at presentation and during COVID-19. The severity of elevated liver chemistries correlates with the outcome of COVID-19. The presence of CLD does not alter the outcome of COVID-19. Further studies are needed to analyse the outcomes of compensated and decompensated liver disease.
© 2020 John Wiley & Sons Ltd.
Figures

Comment in
-
Letter: elevated liver enzymes and outcome in COVID-19.Aliment Pharmacol Ther. 2020 Oct;52(7):1233-1234. doi: 10.1111/apt.16010. Aliment Pharmacol Ther. 2020. PMID: 33016552 No abstract available.
-
Pattern of liver function test variations in COVID-19 infection & its clinical significance: A study from a dedicated COVID-19 tertiary care centre from India.Indian J Med Res. 2022 Sep;156(3):484-499. doi: 10.4103/ijmr.ijmr_1468_21. Indian J Med Res. 2022. PMID: 36751745 Free PMC article.
References
-
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406‐460. - PubMed
-
- Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta‐analysis. Liver Int. 2020;40:1316‐1320. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
