One year follow-up Assessment of Patients Included in the Brazilian Registry of Acute Coronary Syndromes (ACCEPT)
- PMID: 32638905
- PMCID: PMC8416119
- DOI: 10.36660/abc.20190879
One year follow-up Assessment of Patients Included in the Brazilian Registry of Acute Coronary Syndromes (ACCEPT)
Abstract
Background There is lack of prospective data on evolution within one year of acute coronary syndromes (ACS) in a representative population of Brazilian patients. Objectives To assess the prescription of evidence-based therapies, the incidence of severe outcomes and the predictors for these outcomes in a multicenter Brazilian registry of ACS patients. Methods The ACCEPT is a prospective observational study, which included patients hospitalized with a diagnostic of ACS in 47 Brazilian hospitals. The patients were followed for a 1 year and data were collected on the medical prescription and the occurrence of major cardiovascular events (cardiovascular mortality, reinfarction and cerebrovascular accident - CVA). Values of p < 0.05 were considered statistically significant. Results A total of 5,047 patients were included in this registry from August 2010 to April 2014. The diagnosis of ACS was confirmed in 4,782 patients (94.7%) and, among those, the most frequent diagnosis was ACS with ST segment elevation (35.8%). The rate of major cardiovascular events was 13.6 % within 1 year. Adherence to prescription of evidence-based therapy at admission was of 62.1%. Age, public service, acute myocardial infarction, CVA, renal failure, diabetes and quality of therapy were associated independently with the occurrence of major cardiovascular events. Conclusions During the one-year follow-up of the ACCEPT registry, more than 10% of the patients had major cardiovascular events and this rate ranged according with the quality of therapy. Strategies must be elaborated to improve the use of evidence-based therapies to minimize the cardiovascular events among the Brazilian population. (Arq Bras Cardiol. 2020; 114(6):995-1003).
Conflict of interest statement
Potencial conflito de interesses
Pedro Gabriel Melo de Barros e Silva declara ter recebido honorários e bolsas de pesquisa da Pfizer, Roche Diagnostics e Bayer. Otavio Berwanger declara ter recebido bolsas de pesquisa e honorários pessoais da ASTRA Zeneca , Bayer e Boehringer Ingelheim; Bolsas da Amgen e Roche Diagnosis; e honorários pessoais da Novo Nordisk e Novartis.
Figures
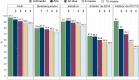



References
-
- 1. World Health Organization (WHO).Cardiovascular Diseases. [Internet]. [Cited in 2019 Dec 12]Available from: http://www.who.int/cardiovascular_diseases/resources/atlas/en/ .
-
- 2. Brasil. Ministério da Saúde. [Cited in 2020 Jan 23]. Available from: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?idb2011/c08.def
-
- 3. Kochanek KD, Xu JQ, Murphy SL, Minino AM, Kung HC. Deaths: preliminary data for 2009. Natl Vital Stat Rep.2011;59(4):1-51. - PubMed
-
- 4. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498-504. - PubMed
-
- 5. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480-6.. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

