Concentration-dependent mortality of chloroquine in overdose
- PMID: 32639233
- PMCID: PMC7417172
- DOI: 10.7554/eLife.58631
Concentration-dependent mortality of chloroquine in overdose
Abstract
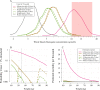
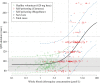
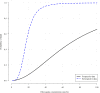
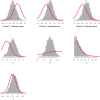
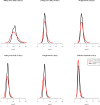
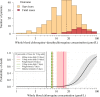
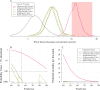
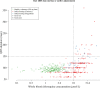
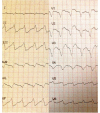
Hydroxychloroquine and chloroquine are used extensively in malaria and rheumatological conditions, and now in COVID-19 prevention and treatment. Although generally safe they are potentially lethal in overdose. In-vitro data suggest that high concentrations and thus high doses are needed for COVID-19 infections, but as yet there is no convincing evidence of clinical efficacy. Bayesian regression models were fitted to survival outcomes and electrocardiograph QRS durations from 302 prospectively studied French patients who had taken intentional chloroquine overdoses, of whom 33 died (11%), and 16 healthy volunteers who took 620 mg base chloroquine single doses. Whole blood concentrations of 13.5 µmol/L (95% credible interval 10.1-17.7) were associated with 1% mortality. Prolongation of ventricular depolarization is concentration-dependent with a QRS duration >150 msec independently highly predictive of mortality in chloroquine self-poisoning. Pharmacokinetic modeling predicts that most high dose regimens trialled in COVID-19 are unlikely to cause serious cardiovascular toxicity.
Keywords: bayesian; chloroquine; human; human biology; medicine; overdose; pharmacodynamics; pharmacokinetics.
Plain language summary
Hydroxychloroquine and chloroquine are closely-related drugs used for the treatment of malaria and rheumatological conditions, such as lupus. Laboratory tests have indicated that these drugs could also be used against the virus that causes COVID-19. Given the urgent need, these drugs have been fast-tracked into large-scale clinical trials, bypassing the usual stages that would provide estimates for suitable dosage. The dosage is a critical factor in a clinical trial: too low and the drug will not have an effect, too high and the side effects may counteract any potential benefits. Laboratory tests suggest that higher doses of chloroquine or hydroxychloroquine are needed for treating COVID-19 compared to malaria or lupus. However, there are concerns about the high doses used in some trials, as the drugs can have lethal side effects. Indeed, chloroquine has been used extensively in suicide attempts, particularly in France. To address these concerns, Watson et al. set out to determine the highest dosage of chloroquine (and thus of hydroxychloroquine, approximately) that does not cause unacceptable side effects. First, data was analysed regarding the concentration of chloroquine in the blood of 302 patients who had intentionally overdosed on the drug, since this concentration is tightly correlated with their risk of death. Watson et al. used a statistical model to calculate the maximal chloroquine concentration in a person’s blood associated with a one per cent risk of death. This is taken to be the threshold above which any potential benefit of chloroquine treatment would be outweighed by the possibility of lethal toxicity. Watson et al. also estimated the relationship between chloroquine concentrations and changes in electrocardiogram patterns, which record the electrical activity of the heart. This makes it possible to determine whether a high dose of chloroquine has led to dangerous levels in the blood. Using a mathematical model of how chloroquine is metabolised, Watson et al. predicted that most of the trials that tested chloroquine as a treatment for COVID-19 did not reach the calculated threshold concentration. An exception was the CloroCovid-19 trial in Brazil, which was stopped early because people in the higher dosage group suffered more heart problems and died in greater numbers than those in the lower dosage group. Two large randomised trials, RECOVERY and SOLIDARITY, have shown no benefit of hydroxychloroquine or chloroquine in the treatment of COVID-19, changing clinical practice worldwide. Both of these trials used high doses resulting in higher hydroxychloroquine or chloroquine concentrations than normally observed in the treatment of malaria or rheumatological conditions. The results from Watson et al demonstrate that the lack of benefit seen in these two large clinical trials is not due to the drug dosage being too high.
© 2020, Watson et al.
Conflict of interest statement
JW, JT, RH, FB, BM, JC, NW No competing interests declared
Figures



References
-
- Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG, CloroCovid-19 Team Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infection: a randomized clinical trial. JAMA Network Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. - DOI - PubMed
-
- Bourré-Tessier J, Urowitz MB, Clarke AE, Bernatsky S, Krantz MJ, Huynh T, Joseph L, Belisle P, Bae SC, Hanly JG, Wallace DJ, Gordon C, Isenberg D, Rahman A, Gladman DD, Fortin PR, Merrill JT, Romero-Diaz J, Sanchez-Guerrero J, Fessler B, Alarcón GS, Steinsson K, Bruce IN, Ginzler E, Dooley MA, Nived O, Sturfelt G, Kalunian K, Ramos-Casals M, Petri M, Zoma A, Pineau CA. Electrocardiographic findings in systemic lupus erythematosus: data from an international inception cohort. Arthritis Care & Research. 2015;67:128–135. doi: 10.1002/acr.22370. - DOI - PubMed
-
- Chan XHS, Win YN, Mawer LJ, Tan JY, Brugada J, White NJ. Risk of sudden unexplained death after use of dihydroartemisinin-piperaquine for malaria: a systematic review and bayesian meta-analysis. The Lancet Infectious Diseases. 2018;18:913–923. doi: 10.1016/S1473-3099(18)30297-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

