Tuberculosis preventive treatment opportunities at antiretroviral therapy initiation and follow-up visits
- PMID: 32639479
- PMCID: PMC7316437
- DOI: 10.5588/pha.19.0056
Tuberculosis preventive treatment opportunities at antiretroviral therapy initiation and follow-up visits
Abstract
Setting: Twenty-two clinics providing HIV care and treatment in Botswana where tuberculosis (TB) and HIV comorbidity is as high as 49%.
Objectives: To assess eligibility of TB preventive treatment (TPT) at antiretroviral therapy (ART) initiation and at four follow-up visits (FUVs), and to describe the TB prevalence and associated factors at baseline and yield of TB diagnoses at each FUV.
Design: A prospective study of routinely collected data on people living with HIV (PLHIV) enrolled into care for the Xpert® MTB/RIF Package Rollout Evaluation Study between 2012 and 2015.
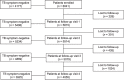
Results: Of 6041 PLHIV initiating ART, eligibility for TPT was 69% (4177/6041) at baseline and 93% (5408/5815); 95% (5234/5514); 96% (4869/5079); and 97% (3925/4055) at FUV1, FUV2, FUV3, and FUV4, respectively. TB prevalence at baseline was 11% and 2%, 3%, 3% and 6% at each subsequent FUV. At baseline, independent risk factors for prevalent TB were CD4 <200 cells/mm3 (aOR = 1.4, P = 0.030); anemia (aOR = 2.39, P < 0.001); cough (aOR = 11.21, P < 0.001); fever (aOR = 2.15, P = 0.001); and weight loss (aOR = 2.60, P = 0.002).
Conclusion: Eligibility for TPT initiation is higher at visits post-ART initiation, while most cases of active TB were identified at ART initiation. Missed opportunities for TB further compromises TB control effort among PLHIV in Botswana.
Marco de referencia: Veintidós consultorios que prestan atención y tratamiento relacionados con la infección por el virus de la inmunodeficiencia humana (VIH) en Botswana, donde la comorbilidad por tuberculosis (TB) e infección por el VIH puede alcanzar 49%.
Objetivos: Evaluar los criterios para recibir el tratamiento preventivo de la TB (TPT) durante las consultas de iniciación y seguimiento del tratamiento antirretrovírico (TAR) y describir la prevalencia de TB y los factores asociados en el momento del inicio y el rendimiento del diagnóstico de TB en cada cita de seguimiento del TAR.
Método: Fue este un estudio prospectivo de los datos obtenidos sistemáticamente en las personas con infección por el VIH (PLHIV), inscritas en la atención para el estudio de evaluación del despliegue de la prueba Xpert® MTB/RIF del 2012 al 2015.
Resultados: De los 6041 PLHIV que iniciaron el TAR, 69% (4177/6041) cumplía los criterios para recibir el TPT al comienzo; 93% (5408/5815) en la primera consulta de seguimiento; 95% (5234/5514) en la segunda; 96% (4869/5079) en la tercera; y 97% (3925/4075) en la cuarta cita de seguimiento. La prevalencia inicial de TB fue 11% y durante el seguimiento fue 2%, 3%, 3% y 6%, respectivamente. Al comienzo del TAR, los factores de riesgo independientes de diagnóstico de TB fueron una cifra de linfocitos CD4 <200 células/mm3 (aOR 1,4; P = 0,030), la anemia (aOR 2,39; P < 0,001), la tos (aOR 11,21; P = <0,001), la fiebre (aOR 2,15; P = 0,001) y la pérdida de peso (aOR 2,60; P = 0,002).
Conclusión: Los pacientes cumplen las condiciones para recibir el TPT con mayor frecuencia en las consultas posteriores al comienzo del TAR, pero la mayoría de los casos de TB activa se detecta al iniciarlo. Las oportunidades desaprovechadas para detectar casos de TB dificultan aún más el control de esta enfermedad en las PLHIV en Botswana.
Keywords: HIV; TB; infection; preventive; treatment.
© 2020 The Union.
Conflict of interest statement
Conflicts of interest: none declared.
Figures

References
-
- World Health Organization. Global tuberculosis report, 2018. Geneva, Switzerland: WHO; 2018.
-
- Ansari A, Kombe H, Kenyon A et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6(1):55–63. - PubMed
-
- World Health Organization. Global tuberculosis report, 2019. Geneva, Switzerland: WHO; 2019.
LinkOut - more resources
Full Text Sources
Research Materials

