A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenström's macroglobulinemia
- PMID: 32639651
- PMCID: PMC7469793
- DOI: 10.1111/cas.14561
A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenström's macroglobulinemia
Erratum in
-
Correction.Cancer Sci. 2021 Apr;112(4):1669. doi: 10.1111/cas.14818. Cancer Sci. 2021. PMID: 33811719 Free PMC article. No abstract available.
Abstract
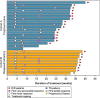
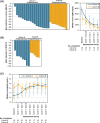
Tirabrutinib is a second-generation Bruton's tyrosine kinase inhibitor with greater selectivity than ibrutinib. Here, we conducted a multicenter, phase II study of tirabrutinib in patients with treatment-naïve (Cohort A) or with relapsed/refractory (Cohort B) Waldenström's macroglobulinemia (WM). Patients were treated with tirabrutinib 480 mg once daily. The primary endpoint was major response rate (MRR; ≥ partial response). Secondary endpoints included overall response rate (ORR; ≥ minor response), time to major response (TTMR), progression-free survival (PFS), overall survival (OS), and safety. In total, 27 patients (18 in Cohort A; 9 in Cohort B) were enrolled. The median age was 71 y, and the median serum immunoglobulin M level was 3600 mg/dL. Among the patients, 96.2% had the MYD88L265P mutation. MRR and ORR were 88.9% and 96.3%, respectively (Cohort A: MRR, 88.9%; ORR, 94.4%; Cohort B: MRR, 88.9%; ORR, 100%). Median TTMR was 1.87 mo. PFS and OS were not reached with a median follow-up of 6.5 and 8.3 mo for Cohorts A and B, respectively. The most common adverse events (AEs) were rash (44.4%), neutropenia (25.9%), and leukopenia (22.2%), with most AEs classified as grade 1 or 2. Grade ≥ 3 AEs included neutropenia (11.1%), lymphopenia (11.1%), and leukopenia (7.4%). No grade 5 AEs were noted. All bleeding events were grade 1; none were associated with drug-related atrial fibrillation or hypertension. Although the follow-up duration was relatively short, the study met the primary endpoint. Therefore, tirabrutinib monotherapy is considered to be highly effective for both untreated and relapsed/refractory WM with a manageable safety profile. (JapicCTI-173646).
Keywords: B-cell malignancy; BTK inhibitor; Japanese; Waldenström’s macroglobulinemia; tirabrutinib.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
N. Sekiguchi has received research funding from Ono Pharmaceutical. W. Munakata has received research funding from Ono Pharmaceutical. H. Handa has received research funding from Ono Pharmaceutical. H. Shibayama has received honoraria from Takeda, Novartis, Celgene, Janssen, Chugai, and Kyowa Kirin; research funding from Janssen, Ono Pharmaceutical, Celgene, Novartis, Sanofi, AstraZeneca, AbbVie, and Chugai; and scholarship endowment from Astellas, Teijin, Shionogi, Eisai, Sanofi, Taiho, and Nippon Shinyaku. Y. Terui has received honoraria from Chugai, Celgene, Bristol‐Myers Squibb, Novartis, Janssen, and Ono Pharmaceutical; and research funding from Bristol‐Myers Squibb. N. Fukuhara has received honoraria from Chugai pharma and Kyowa Kirin; and research funding from AbbVie, Bayer, Chugai, Eisai, Gilead Sciences, Incyte, Ono Pharmaceutical, and Solasia. S. Iida has received honoraria from Ono Pharmaceutical, Takeda, Janssen, Celgene, Bristol‐Myers Squibb, Daiichi Sankyo, and Sanofi; and research funding from Ono Pharmaceutical, Takeda, Bristol‐Myers Squibb, MSD, Janssen, AbbVie, Kyowa Kirin, Chugai, and Sanofi. K. Izutsu has received honoraria from Kyowa Kirin and Eisai; and research funding from Celgene, Chugai, Novartis, Ono, Pharmaceutical, Bayer, Zenyaku Kogyo, Kyowa Kirin, AstraZeneca, Eisai, Incyte, AbbVie, Symbio, Janssen, and Yakult. The other authors have no relationships to disclose. This work was supported by Ono Pharmaceutical. The study sponsor was involved in study design, writing of the report, and in the decision to submit the article for publication. Study drug was provided by Ono Pharmaceutical. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Figures

References
-
- Swerdlow S, Cook J, Sohani A, et al. Lymphoplasmacytic lymphoma In: Swerdlow S, Campo E, Harris N, eds. WHO Classification of Tumour of Hematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2017:232–235.
-
- Owen RG, Treon SP, Al‐Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–115. - PubMed
-
- Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenström’s macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5:e299–e309. - PubMed
-
- Olszewski AJ, Treon SP, Castillo JJ. Application and Outcomes of Bendamustine‐ or Bortezomib‐Based Therapy for Waldenstrom’s Macroglobulinemia [abstract]. Blood. 2017;130(Supplement 1):348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

