Poly(ADP-Ribose) Polymerase Inhibition in Acute Lung Injury. A Reemerging Concept
- PMID: 32640172
- PMCID: PMC7605157
- DOI: 10.1165/rcmb.2020-0188TR
Poly(ADP-Ribose) Polymerase Inhibition in Acute Lung Injury. A Reemerging Concept
Abstract
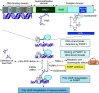
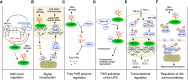
PARP1, the major isoform of a family of ADP-ribosylating enzymes, has been implicated in the regulation of various biological processes including DNA repair, gene transcription, and cell death. The concept that PARP1 becomes activated in acute lung injury (ALI) and that pharmacological inhibition or genetic deletion of this enzyme can provide therapeutic benefits emerged over 20 years ago. The current article provides an overview of the cellular mechanisms involved in the pathogenetic roles of PARP1 in ALI and provides an overview of the preclinical data supporting the efficacy of PARP (poly[ADP-ribose] polymerase) inhibitors. In recent years, several ultrapotent PARP inhibitors have been approved for clinical use (for the therapy of various oncological diseases): these newly-approved PARP inhibitors were recently reported to show efficacy in animal models of ALI. These observations offer the possibility of therapeutic repurposing of these inhibitors for patients with ALI. The current article lays out a potential roadmap for such repurposing efforts. In addition, the article also overviews the scientific basis of potentially applying PARP inhibitors for the experimental therapy of viral ALI, such as coronavirus disease (COVID-19)-associated ALI.
Keywords: cell death; coronavirus; cytokines; inflammation; olaparib.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

