Pharmacokinetics and Metabolism of Liposome-Encapsulated 2,4,6-Trihydroxygeranylacetophenone in Rats Using High-Resolution Orbitrap Liquid Chromatography Mass Spectrometry
- PMID: 32640512
- PMCID: PMC7412073
- DOI: 10.3390/molecules25133069
Pharmacokinetics and Metabolism of Liposome-Encapsulated 2,4,6-Trihydroxygeranylacetophenone in Rats Using High-Resolution Orbitrap Liquid Chromatography Mass Spectrometry
Abstract
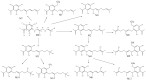
2,4,6-trihydroxy-3-geranylacetophenone (tHGA) is a bioactive compound that shows excellent anti-inflammatory properties. However, its pharmacokinetics and metabolism have yet to be evaluated. In this study, a sensitive LC-HRMS method was developed and validated to quantify tHGA in rat plasma. The method showed good linearity (0.5-80 ng/mL). The accuracy and precision were within 10%. Pharmacokinetic investigations were performed on three groups of six rats. The first two groups were given oral administrations of unformulated and liposome-encapsulated tHGA, respectively, while the third group received intraperitoneal administration of liposome-encapsulated tHGA. The maximum concentration (Cmax), the time required to reach Cmax (tmax), elimination half-life (t1/2) and area under curve (AUC0-24) values for intraperitoneal administration were 54.6 ng/mL, 1.5 h, 6.7 h, and 193.9 ng/mL·h, respectively. For the oral administration of unformulated and formulated tHGA, Cmax values were 5.4 and 14.5 ng/mL, tmax values were 0.25 h for both, t1/2 values were 6.9 and 6.6 h, and AUC0-24 values were 17.6 and 40.7 ng/mL·h, respectively. The liposomal formulation improved the relative oral bioavailability of tHGA from 9.1% to 21.0% which was a 2.3-fold increment. Further, a total of 12 metabolites were detected and structurally characterized. The metabolites were mainly products of oxidation and glucuronide conjugation.
Keywords: LC-HRMS method validation; liposomes; metabolism; pharmacokinetics; trihydroxygeranylacetophenone.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Shaari K., Suppaiah V., Wai L.K., Stanslas J., Tejo B.A., Israf D.A., Abas F., Ismail I.S., Shuaib N.H., Zareen S. Bioassay-guided identification of an anti-inflammatory prenylated acylphloroglucinol from Melicope ptelefolia and molecular insights into its interaction with 5-lipoxygenase. Bioorg. Med. Chem. 2011;19:6340–6347. doi: 10.1016/j.bmc.2011.09.001. - DOI - PubMed
-
- Ismail N., Jambari N.N., Zareen S., Akhtar M.N., Shaari K., Zamri-Saad M., Tham C.L., Sulaiman M.R., Lajis N.H., Israf D.A. A geranyl acetophenone targeting cysteinyl leukotriene synthesis prevents allergic airway inflammation in ovalbumin-sensitized mice. Toxicol. Appl. Pharmacol. 2012;259:257–262. doi: 10.1016/j.taap.2012.01.003. - DOI - PubMed
-
- Chong Y.J., Musa N.F., Ng C.H., Shaari K., Israf D.A., Tham C.L. Barrier protective effects of 2,4,6-trihydroxy-3-geranyl acetophenone on lipopolysaccharides-stimulated inflammatory responses in human umbilical vein endothelial cells. J. Ethnopharmacol. 2016;192:248–255. doi: 10.1016/j.jep.2016.07.032. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

