Candesartan Cilexetil In Vitro-In Vivo Correlation: Predictive Dissolution as a Development Tool
- PMID: 32640620
- PMCID: PMC7408357
- DOI: 10.3390/pharmaceutics12070633
Candesartan Cilexetil In Vitro-In Vivo Correlation: Predictive Dissolution as a Development Tool
Abstract
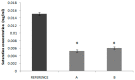
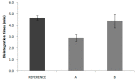
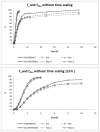
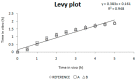
The main objective of this investigation was to develop an in vitro-in vivo correlation (IVIVC) for immediate release candesartan cilexetil formulations by designing an in vitro dissolution test to be used as development tool. The IVIVC could be used to reduce failures in future bioequivalence studies. Data from two bioequivalence studies were scaled and combined to obtain the dataset for the IVIVC. Two-step and one-step approaches were used to develop the IVIVC. Experimental solubility and permeability data confirmed candesartan cilexetil. Biopharmaceutic Classification System (BCS) class II candesartan average plasma profiles were deconvoluted by the Loo-Riegelman method to obtain the oral fractions absorbed. Fractions dissolved were obtained in several conditions in USP II and IV apparatus and the results were compared calculating the f2 similarity factor. Levy plot was constructed to estimate the time scaling factor and to make both processes, dissolution and absorption, superimposable. The in vitro dissolution experiment that reflected more accurately the in vivo behavior of the products of candesartan cilexetil employed the USP IV apparatus and a three-step pH buffer change, from 1.2 to 4.5 and 6.8, with 0.2% of Tween 20. This new model was able to predict the in vivo differences in dissolution and it could be used as a risk-analysis tool for formulation selection in future bioequivalence trials.
Keywords: BCS; IVIVC; bioequivalence; candesartan cilexetil; predictive in vivo-dissolution.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
-
- EMA . Guideline on the Investigation of Bioequivalence. European Medicines Agency; London, UK: 2010. Doc. Ref.: CPMP/EWP/QWP/1401/98. - PubMed
-
- Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry. FDA; Silver Spring, MD, USA: 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

