Development of Artificial Plasma Membranes Derived Nanovesicles Suitable for Drugs Encapsulation
- PMID: 32640653
- PMCID: PMC7408059
- DOI: 10.3390/cells9071626
Development of Artificial Plasma Membranes Derived Nanovesicles Suitable for Drugs Encapsulation
Abstract
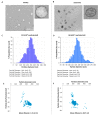
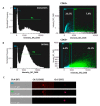
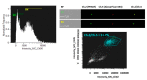
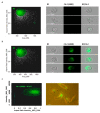
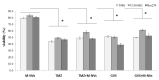
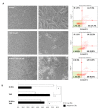
Extracellular vesicles (EVs) are considered as promising nanoparticle theranostic tools in many pathological contexts. The increasing clinical employment of therapeutic nanoparticles is contributing to the development of a new research area related to the design of artificial EVs. To this aim, different approaches have been described to develop mimetic biologically functional nanovescicles. In this paper, we suggest a simplified procedure to generate plasma membrane-derived nanovesicles with the possibility to efficiently encapsulate different drugs during their spontaneously assembly. After physical and molecular characterization by Tunable Resistive Pulse Sensing (TRPS) technology, transmission electron microscopy, and flow cytometry, as a proof of principle, we have loaded into mimetic EVs the isoquinoline alkaloid Berberine chloride and the chemotherapy compounds Temozolomide or Givinostat. We demonstrated the fully functionality of these nanoparticles in drug encapsulation and cell delivery, showing, in particular, a similar cytotoxic effect of direct cell culture administration of the anticancer drugs. In conclusion, we have documented the possibility to easily generate scalable nanovesicles with specific therapeutic cargo modifications useful in different drug delivery contexts.
Keywords: drugs-delivery; extracellular vesicles; nanomedicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

