Effects of DNA Damage and Oxidative Stress in Human Bronchial Epithelial Cells Exposed to PM2.5 from Beijing, China, in Winter
- PMID: 32640694
- PMCID: PMC7369897
- DOI: 10.3390/ijerph17134874
Effects of DNA Damage and Oxidative Stress in Human Bronchial Epithelial Cells Exposed to PM2.5 from Beijing, China, in Winter
Abstract
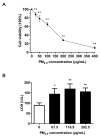
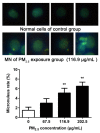
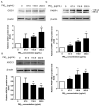
Epidemiological studies have corroborated that respiratory diseases, including lung cancer, are related to fine particulate matter (<2.5 μm) (PM2.5) exposure. The toxic responses of PM2.5 are greatly influenced by the source of PM2.5. However, the effects of PM2.5 from Beijing on bronchial genotoxicity are scarce. In the present study, PM2.5 from Beijing was sampled and applied in vitro to investigate its genotoxicity and the mechanisms behind it. Human bronchial epithelial cells 16HBE were used as a model for exposure. Low (67.5 μg/mL), medium (116.9 μg/mL), and high (202.5 μg/mL) doses of PM2.5 were used for cell exposure. After PM2.5 exposure, cell viability, oxidative stress markers, DNA (deoxyribonucleic acid) strand breaks, 8-OH-dG levels, micronuclei formation, and DNA repair gene expression were measured. The results showed that PM2.5 significantly induced cytotoxicity in 16HBE. Moreover, the levels of reactive oxygen species (ROS), malondialdehyde (MDA), and cellular heme oxygenase (HO-1) were increased, and the level of glutathione (GSH) was decreased, which represented the occurrence of severe oxidative stress in 16HBE. The micronucleus rate was elevated, and DNA damage occurred as indicators of the comet assay, γ-H2AX and 8-OH-dG, were markedly enhanced by PM2.5, accompanied by the influence of 8-oxoguanine DNA glycosylase (OGG1), X-ray repair cross-complementing gene 1 (XRCC1), and poly (ADP-ribose) polymerase-1 (PARP1) expression. These results support the significant role of PM2.5 genotoxicity in 16HBE cells, which may occur through the combined effect on oxidative stress and the influence of DNA repair genes.
Keywords: DNA damage; DNA repair gene; PM2.5; human bronchial epithelial cells; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Effects of Ambient Atmospheric PM2.5, 1-Nitropyrene and 9-Nitroanthracene on DNA Damage and Oxidative Stress in Hearts of Rats.Cardiovasc Toxicol. 2019 Apr;19(2):178-190. doi: 10.1007/s12012-018-9488-5. Cardiovasc Toxicol. 2019. PMID: 30421179
-
Interleukins 6/8 and cyclooxygenase-2 release and expressions are regulated by oxidative stress-JAK2/STAT3 signaling pathway in human bronchial epithelial cells exposed to particulate matter ≤2.5 μm.J Appl Toxicol. 2020 Sep;40(9):1210-1218. doi: 10.1002/jat.3977. Epub 2020 Mar 24. J Appl Toxicol. 2020. PMID: 32212198
-
Assessment of DNA Damage and Cell Senescence in Corneal Epithelial Cells Exposed to Airborne Particulate Matter (PM2.5) Collected in Guangzhou, China.Invest Ophthalmol Vis Sci. 2016 Jun 1;57(7):3093-102. doi: 10.1167/iovs.15-18839. Invest Ophthalmol Vis Sci. 2016. PMID: 27286367
-
Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells.PLoS One. 2015 Sep 18;10(9):e0138267. doi: 10.1371/journal.pone.0138267. eCollection 2015. PLoS One. 2015. PMID: 26382838 Free PMC article.
-
A review of respirable fine particulate matter (PM2.5)-induced brain damage.Front Mol Neurosci. 2022 Sep 7;15:967174. doi: 10.3389/fnmol.2022.967174. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157076 Free PMC article. Review.
Cited by
-
Update in Association between Lung Cancer and Air Pollution.Tuberc Respir Dis (Seoul). 2025 Apr;88(2):228-236. doi: 10.4046/trd.2024.0092. Epub 2024 Dec 11. Tuberc Respir Dis (Seoul). 2025. PMID: 39659117 Free PMC article.
-
The Cytotoxic Effects of Fine Particulate Matter (PM2.5) from Different Sources at the Air-Liquid Interface Exposure on A549 Cells.Toxics. 2023 Dec 25;12(1):21. doi: 10.3390/toxics12010021. Toxics. 2023. PMID: 38250977 Free PMC article.
-
The impact of social and environmental factors on cancer biology in Black Americans.Cancer Causes Control. 2023 Mar;34(3):191-203. doi: 10.1007/s10552-022-01664-w. Epub 2022 Dec 23. Cancer Causes Control. 2023. PMID: 36562901 Review.
-
Oxidative damage to DNA, expression of Mt-1, and activation of repair mechanisms induced by vanadium trioxide in cultures of human lymphocytes.Toxicol Rep. 2025 Jan 13;14:101909. doi: 10.1016/j.toxrep.2025.101909. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 39897397 Free PMC article.
-
Calling Attention to the Role of Race-Driven Societal Determinants of Health on Aggressive Tumor Biology: A Focus on Black Americans.JCO Oncol Pract. 2022 Jan;18(1):15-22. doi: 10.1200/OP.21.00297. Epub 2021 Jul 13. JCO Oncol Pract. 2022. PMID: 34255546 Free PMC article.
References
-
- Gualtieri M., Grollino M.G., Consales C., Costabile F., Manigrasso M., Avino P., Aufderheide M., Cordelli E., Di Liberto L., Petralia E., et al. Is it the time to study air pollution effects under environmental conditions? A case study to support the shift of in vitro toxicology from the bench to the field. Chemosphere. 2018;207:552–564. doi: 10.1016/j.chemosphere.2018.05.130. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

