Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis
- PMID: 32640720
- PMCID: PMC7408905
- DOI: 10.3390/jcm9072132
Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis
Abstract
Background: Neoadjuvant therapy (NAT) represents a paradigm shift in the management of patients with pancreatic ductal adenocarcinoma (PDAC) with perceived benefits including a higher R0 rate. However, it is unclear whether NAT affects the sites and patterns of recurrence after surgery. This review seeks to compare sites and patterns of recurrence after resection between patients undergoing upfront surgery (US) or after NAT.
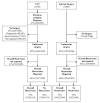
Methods: The EMBASE, SCOPUS, PubMed, and Cochrane library databases were systematically searched to identify eligible studies that compare recurrence patterns between patients who had NAT (followed by resection) with those that had US. The primary outcome included site-specific recurrence.
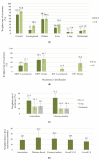
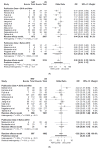
Results: 26 articles were identified including 4986 patients who underwent resection. Borderline resectable pancreatic cancer (BRPC, 47% 1074/2264) was the most common, followed by resectable pancreatic cancer (RPC 42%, 949/2264). The weighted overall recurrence rates were lower among the NAT group, 63.4% vs. 74% (US) (OR 0.67 (CI 0.52-0.87), p = 0.006). The overall weighted locoregional recurrence rate was lower amongst patients who received NAT when compared to US (12% vs 27% OR 0.39 (CI 0.22-0.70), p = 0.004). In BRPC, locoregional recurrence rates improved with NAT (NAT 25.8% US 37.7% OR 0.62 (CI 0.44-0.87), p = 0.007). NAT was associated with a lower weighted liver recurrence rate (NAT 19.4% US 30.1% OR 0.55 (CI 0.34-0.89), p = 0.023). Lung and peritoneal recurrence rates did not differ between NAT and US cohorts (p = 0.705 and p = 0.549 respectively). NAT was associated with a significantly longer weighted mean time to first recurrence 18.8 months compared to US (15.7 months) (OR 0.18 (CI 0.05-0.32), p = 0.015).
Conclusion: NAT was associated with lower overall recurrence rate and improved locoregional disease control particularly for those with BRPC. Although the burden of liver metastases was less, there was no overall effect upon distant metastatic disease.
Keywords: neoadjuvant chemotherapy; pancreatic ductal adenocarcinoma; pancreatic surgery; recurrence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sergeant G., Ectors N., Van Steenbergen W., Aerts R., Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2008;35:600–604. - PubMed
LinkOut - more resources
Full Text Sources

