Effects of Sotagliflozin Combined with Intensive Insulin Therapy in Young Adults with Poorly Controlled Type 1 Diabetes: The JDRF Sotagliflozin Study
- PMID: 32640846
- PMCID: PMC7864092
- DOI: 10.1089/dia.2020.0079
Effects of Sotagliflozin Combined with Intensive Insulin Therapy in Young Adults with Poorly Controlled Type 1 Diabetes: The JDRF Sotagliflozin Study
Abstract
Background: Young adults with type 1 diabetes (T1D) tend to have higher A1C than older adults and are at increased risk for diabetic ketoacidosis (DKA). Oral adjuncts to insulin have not been previously studied in this population. Methods: In this phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group study, adults aged 18-30 years with T1D and A1C ≥9.0% were randomly assigned to placebo (n = 42) or sotagliflozin 400 mg (n = 43), in addition to insulin for 12 weeks. Insulin doses were adjusted to meet glucose targets (preprandial 80-130 mg/dL, postprandial <180 mg/dL). The primary endpoint was change from baseline in A1C at week 12. Results: From a baseline of 9.8%, mean A1C decreased by 1.0% with placebo and 1.3% with sotagliflozin (-0.4% [95% confidence interval (CI): -0.8 to 0.1]; P = 0.10 vs. placebo). In the prespecified A1C ≤10.0% subgroup, the treatment difference was -0.8% (-1.3 to -0.2; P = 0.006), favoring sotagliflozin. Overall, relative to placebo, postprandial glucose (PPG) decreased by 56.6 mg/dL (-89.7 to -23.6; P < 0.001) and weight decreased by 2.37 kg (-3.5 to -1.2; P < 0.001). More patients achieved an A1C <7.0% with sotagliflozin (16.3%) than placebo (2.4%; P = 0.026). Rates of documented hypoglycemia and severe hypoglycemia were similar between groups. One DKA event occurred with placebo, and none occurred with sotagliflozin. Conclusions: In young adults with T1D and suboptimal glycemic control, sotagliflozin plus insulin for 12 weeks numerically improved A1C and significantly improved A1C goal attainment, PPG, and body weight. Sotagliflozin plus insulin was generally well tolerated without any episodes of DKA (NCT02383940).
Keywords: Adjunctive therapy; Diabetic ketoacidosis; SGLT inhibitors; Sotagliflozin; Type 1 diabetes; Young adults.
Conflict of interest statement
B.W.B. has served as a consultant for Adocia, Eli Lilly and Company, Medtronic, Novo Nordisk, Lexicon Pharmaceuticals, Inc., and Pfizer; has served on speaker's bureaus for Astra Zeneca, Lilly/Boehringer Ingelheim, Janssen, Medtronic, Novo Nordisk, Sanofi, and Senseonics; and owns stock in Aseko; and his employer (Atlanta Diabetes Associates) has received grant and/or research support from Abbott, DexCom, Diasome, GSK, Janssen, Insulet, Lexicon Pharmaceuticals, Inc., Lilly/Boehringer Ingelheim, Mannkind, Medtronic, the National Institutes of Health, Nova Biomedical, Novo Nordisk, Provention Bio, Sanofi, Senseonics, REMD Biotherapeutics, Xeris, and vTv Therapeutics LLC. E.C. is a speaker for Novo Nordisk and serves on the advisory board for Novo Nordisk, MannKind, Adocia, Arecor, and Lexicon. R.P.W. has acted as an advisory board member for Eli Lilly and Medtronic, as a speaker and consultant for Dexcom and Tandem Diabetes Care; has received research support from Bigfoot Biomedical, Dexcom, Eli Lilly, MannKind Corporation, Novo Nordisk, and Tandem Diabetes Care and research funding and other support from Lexicon during the conduct of the study. P.B. is employed by and holds stock in Lexicon Pharmaceuticals, Inc. T.D. has acted as consultant, advisory board member, steering committee member, or speaker for Abbott, Medtronic, Roche, Lexicon, Menarini, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi, Dexcom, and Eli Lilly; has received research grants from Abbott, AstraZeneca, Novo Nordisk, Medtronic, and Sanofi. J.A.K. serves as medical director of McNair Interests, a private equity group with investments in T1D and other chronic illnesses, and he is also an advisor for Sanofi and Lexicon. D.K.M. has received honoraria for clinical trials leadership from GlaxoSmithKline, Janssen, Lexicon, Boehringer Ingelheim, AstraZeneca, Sanofi Aventis, Merck & Co, Pfizer, Novo Nordisk, Esperion, and Lilly USA; consulting fees from AstraZeneca, Merck Sharp & Dohme, GlaxoSmithKline, Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Metavant, Sanofi Aventis, and Afimmune. A.L.P. has participated on advisory boards for Abbott Diabetes Care, Boehringer Ingelheim, Eli Lilly and Company, MannKind, Medscape, Novo Nordisk, Sanofi, and Lexicon; has received research funding from Dexcom and vTv Therapeutics; and has stock options for Mellitus Health, Pendulum Therapeutics, Omaha Health, Stability Health, and Livongo. P.S. and S.S. were employed by Lexicon Pharmaceuticals at the time the study was conducted. P.S. is now employed by Metavant Sciences, Ltd., and S.S. is employed by Immuvant, Inc.
Figures
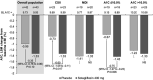
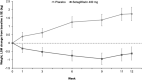
References
-
- Thomas AM, Peterson L, Goldstein D: Problem solving and diabetes regimen adherence by children and adolescents with IDDM in social pressure situations: a reflection of normal development. J Pediatr Psychol 1997;22:541–561 - PubMed
-
- Bode BW, Garg SK: The emerging role of adjunctive noninsulin antihyperglycemic therapy in the management of type 1 diabetes. Endocr Pract 2016;22:220–230 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

