The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine
- PMID: 32640894
- PMCID: PMC7400725
- DOI: 10.1177/1535370220938741
The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine
Abstract
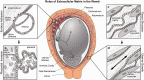
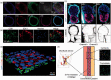
Extracellular matrix in the womb regulates the initiation, progression, and completion of a healthy pregnancy. The composition and physical properties of extracellular matrix in the uterus and at the maternal-fetal interface are remodeled at each gestational stage, while maladaptive matrix remodeling results in obstetric disease. As in vitro models of uterine and placental tissues, including micro-and milli-scale versions of these organs on chips, are developed to overcome the inherent limitations of studying human development in vivo, we can isolate the influence of cellular and extracellular components in healthy and pathological pregnancies. By understanding and recreating key aspects of the extracellular microenvironment at the maternal-fetal interface, we can engineer microphysiological systems to improve assisted reproduction, obstetric disease treatment, and prenatal drug safety.
Keywords: Extracellular matrix; maternal–fetal interface; obstetric diseases; placenta; pregnancy.
Figures

References
-
- Muiznieks LD, Keeley FW. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim Biophys Acta 2013; 1832:866–75 - PubMed
-
- Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn 1993; 43:283–93 - PubMed
-
- Piez KA. History of extracellular matrix: a personal view. Matrix Biol 1997; 16:85–92 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

