Fibulin-1 Integrates Subendothelial Extracellular Matrices and Contributes to Anatomical Closure of the Ductus Arteriosus
- PMID: 32640908
- PMCID: PMC7447190
- DOI: 10.1161/ATVBAHA.120.314729
Fibulin-1 Integrates Subendothelial Extracellular Matrices and Contributes to Anatomical Closure of the Ductus Arteriosus
Abstract
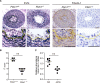
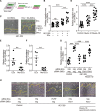
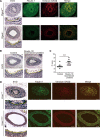
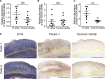
Objective: The ductus arteriosus (DA) is a fetal artery connecting the aorta and pulmonary arteries. Progressive matrix remodeling, that is, intimal thickening (IT), occurs in the subendothelial region of DA to bring anatomic DA closure. IT is comprised of multiple ECMs (extracellular matrices) and migrated smooth muscle cells (SMCs). Because glycoprotein fibulin-1 binds to multiple ECMs and regulates morphogenesis during development, we investigated the role of fibulin-1 in DA closure. Approach and Results: Fibulin-1-deficient (Fbln1-/-) mice exhibited patent DA with hypoplastic IT. An unbiased transcriptome analysis revealed that EP4 (prostaglandin E receptor 4) stimulation markedly increased fibulin-1 in DA-SMCs via phospholipase C-NFκB (nuclear factor κB) signaling pathways. Fluorescence-activated cell sorting (FACS) analysis demonstrated that fibulin-1 binding protein versican was derived from DA-endothelial cells (ECs). We examined the effect of fibulin-1 on directional migration toward ECs in association with versican by using cocultured DA-SMCs and ECs. EP4 stimulation promoted directional DA-SMC migration toward ECs, which was attenuated by either silencing fibulin-1 or versican. Immunofluorescence demonstrated that fibulin-1 and versican V0/V1 were coexpressed at the IT of wild-type DA, whereas 30% of versican-deleted mice lacking a hyaluronan binding site displayed patent DA. Fibulin-1 expression was attenuated in the EP4-deficient mouse (Ptger4-/-) DA, which exhibits patent DA with hypoplastic IT, and fibulin-1 protein administration restored IT formation. In human DA, fibulin-1 and versican were abundantly expressed in SMCs and ECs, respectively.
Conclusions: Fibulin-1 contributes to DA closure by forming an environment favoring directional SMC migration toward the subendothelial region, at least, in part, in combination with EC-derived versican and its binding partner hyaluronan.
Keywords: ductus arteriosus; extracellular matrix; fibulin; mice; neointima; vascular remodeling; versicans.
Conflict of interest statement
None.
Figures


References
-
- Yokoyama U. Prostaglandin E-mediated molecular mechanisms driving remodeling of the ductus arteriosus. Pediatr Int 201557820–827doi: 10.1111/ped.12769 - PubMed
-
- Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, Zea AM, Zhang Y, Sadeghirad B, Thabane L. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA 20183191221–1238doi: 10.1001/jama.2018.1896 - PMC - PubMed
-
- Ito S, Matsuda T, Usuda H, Watanabe S, Kitanishi R, Hanita T, Watanabe T, Adachi O. Surgical ligation for patent ductus arteriosus in extremely premature infants: strategy to reduce their risk of neurodevelopmental impairment. Tohoku J Exp Med 20162407–13doi: 10.1620/tjem.240.7 - PubMed
-
- Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, Sekar K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 2009123e138–e144doi: 10.1542/peds.2008-2418 - PubMed
-
- Clyman RI. Ibuprofen and patent ductus arteriosus. N Engl J Med 2000343728–730doi: 10.1056/NEJM200009073431009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

