Sacubitril/Valsartan in Advanced Heart Failure With Reduced Ejection Fraction: Rationale and Design of the LIFE Trial
- PMID: 32641226
- PMCID: PMC7286640
- DOI: 10.1016/j.jchf.2020.05.005
Sacubitril/Valsartan in Advanced Heart Failure With Reduced Ejection Fraction: Rationale and Design of the LIFE Trial
Erratum in
-
Correction.JACC Heart Fail. 2020 Dec;8(12):1059. doi: 10.1016/j.jchf.2020.10.011. JACC Heart Fail. 2020. PMID: 33272389 Free PMC article. No abstract available.
Abstract
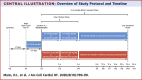
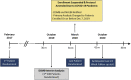
The PARADIGM-HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial reported that sacubitril/valsartan (S/V), an angiotensin receptor-neprilysin inhibitor, significantly reduced mortality and heart failure (HF) hospitalization in HF patients with a reduced ejection fraction (HFrEF). However, fewer than 1% of patients in the PARADIGM-HF study had New York Heart Association (NYHA) functional class IV symptoms. Accordingly, data that informed the use of S/V among patients with advanced HF were limited. The LIFE (LCZ696 in Hospitalized Advanced Heart Failure) study was a 24-week prospective, multicenter, double-blinded, double-dummy, active comparator trial that compared the safety, efficacy, and tolerability of S/V with those of valsartan in patients with advanced HFrEF. The trial planned to randomize 400 patients ≥18 years of age with advanced HF, defined as an EF ≤35%, New York Heart Association functional class IV symptoms, elevated natriuretic peptide concentration (B-type natriuretic peptide [BNP] ≥250 pg/ml or N-terminal pro-B-type natriuretic peptide [NT-proBNP] ≥800 pg/ml), and ≥1 objective finding of advanced HF. Following a 3- to 7-day open label run-in period with S/V (24 mg/26 mg twice daily), patients were randomized 1:1 to S/V titrated to 97 mg/103 mg twice daily versus 160 mg of V twice daily. The primary endpoint was the proportional change from baseline in the area under the curve for NT-proBNP levels measured through week 24. Secondary and tertiary endpoints included clinical outcomes and safety and tolerability. Because of the COVID-19 pandemic, enrollment in the LIFE trial was stopped prematurely to ensure patient safety and data integrity. The primary analysis consists of the first 335 randomized patients whose clinical follow-up examination results were not severely impacted by COVID-19. (Entresto [LCZ696] in Advanced Heart Failure [LIFE STUDY] [HFN-LIFE]; NCT02816736).
Keywords: NYHA functional class IV; heart failure; sacubitril/valsartan; valsartan.
Copyright © 2020 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Should the LIFE Trial Assess Improvement in Ejection Fraction as a Primary Endpoint?JACC Heart Fail. 2020 Nov;8(11):959-960. doi: 10.1016/j.jchf.2020.07.006. JACC Heart Fail. 2020. PMID: 33121709 No abstract available.
References
-
- Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. - PubMed
-
- Yancy C.W., Jessup M., Bozkurt B., et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. - PubMed
-
- Fang J.C., Ewald G.A., Allen L.A., et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. - PubMed
-
- McMurray J.J., Packer M., Desai A.S., et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–1073. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

