Impact of carbohydrate-reduced nutrition in septic patients on ICU: study protocol for a prospective randomised controlled trial
- PMID: 32641340
- PMCID: PMC7348645
- DOI: 10.1136/bmjopen-2020-038532
Impact of carbohydrate-reduced nutrition in septic patients on ICU: study protocol for a prospective randomised controlled trial
Abstract
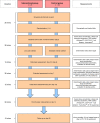
Introduction: Sepsis is defined as detrimental immune response to an infection. This overwhelming reaction often abolishes a normal reconstitution of the immune cell homeostasis that in turn increases the risk for further complications. Recent studies revealed a favourable impact of ketone bodies on resolution of inflammation. Thus, a ketogenic diet may provide an easy-to-apply and cost-effective treatment option potentially alleviating sepsis-evoked harm. This study is designed to assess the feasibility, efficiency and safety of a ketogenic diet in septic patients.
Methods and analysis: This monocentric study is a randomised, controlled and open-label trial, which is conducted on an intensive care unit of a German university hospital. As intervention enteral nutrition with reduced amount of carbohydrates (ketogenic) or standard enteral nutrition (control) is applied. The primary endpoint is the detection of ketone bodies in patients' blood and urine samples. As secondary endpoints, the impact on important safety-relevant issues (eg, glucose metabolism, lactate serum concentration, incidence of metabolic acidosis, thyroid function and 30-day mortality) and the effect on the immune system are analysed.
Ethics and dissemination: The study has received the following approvals: Ethics Committee of the Medical Faculty of Ruhr-University Bochum (No. 18-6557-BR). Results will be made available to critical care survivors, their caregivers, the funders, the critical care societies and other researchers by publication in a peer-reviewed journal.
Trial registration numbers: German Clinical Trial Register (DRKS00017710); Universal Trial Number (U1111-1237-2493).
Keywords: adult intensive & critical care; immunology; intensive & critical care; nutrition & dietetics.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- DRKS/DRKS00017710
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
