Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy
- PMID: 32641768
- PMCID: PMC7343844
- DOI: 10.1038/s41467-020-17186-5
Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy
Erratum in
-
Author Correction: Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy.Nat Commun. 2020 Aug 7;11(1):4041. doi: 10.1038/s41467-020-17979-8. Nat Commun. 2020. PMID: 32769982 Free PMC article.
Abstract
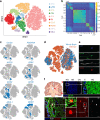
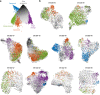
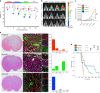
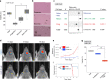
Cancer stem cells are critical for cancer initiation, development, and treatment resistance. Our understanding of these processes, and how they relate to glioblastoma heterogeneity, is limited. To overcome these limitations, we performed single-cell RNA sequencing on 53586 adult glioblastoma cells and 22637 normal human fetal brain cells, and compared the lineage hierarchy of the developing human brain to the transcriptome of cancer cells. We find a conserved neural tri-lineage cancer hierarchy centered around glial progenitor-like cells. We also find that this progenitor population contains the majority of the cancer's cycling cells, and, using RNA velocity, is often the originator of the other cell types. Finally, we show that this hierarchal map can be used to identify therapeutic targets specific to progenitor cancer stem cells. Our analyses show that normal brain development reconciles glioblastoma development, suggests a possible origin for glioblastoma hierarchy, and helps to identify cancer stem cell-specific targets.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

