Fitness trade-offs incurred by ovary-to-gut steroid signalling in Drosophila
- PMID: 32641829
- PMCID: PMC7442704
- DOI: 10.1038/s41586-020-2462-y
Fitness trade-offs incurred by ovary-to-gut steroid signalling in Drosophila
Abstract
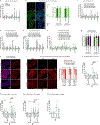
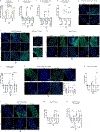
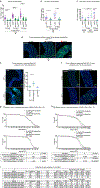
Sexual dimorphism arises from genetic differences between male and female cells, and from systemic hormonal differences1-3. How sex hormones affect non-reproductive organs is poorly understood, yet highly relevant to health given the sex-biased incidence of many diseases4. Here we report that steroid signalling in Drosophila from the ovaries to the gut promotes growth of the intestine specifically in mated females, and enhances their reproductive output. The active ovaries of the fly produce the steroid hormone ecdysone, which stimulates the division and expansion of intestinal stem cells in two distinct proliferative phases via the steroid receptors EcR and Usp and their downstream targets Broad, Eip75B and Hr3. Although ecdysone-dependent growth of the female gut augments fecundity, the more active and more numerous intestinal stem cells also increase female susceptibility to age-dependent gut dysplasia and tumorigenesis, thus potentially reducing lifespan. This work highlights the trade-offs in fitness traits that occur when inter-organ signalling alters stem-cell behaviour to optimize organ size.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures






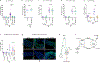


Comment in
-
Sexual Dimorphism: Ecdysone Modulates Sex Differences in the Gut.Curr Biol. 2020 Nov 2;30(21):R1327-R1330. doi: 10.1016/j.cub.2020.08.088. Curr Biol. 2020. PMID: 33142105
References
EXTENDED REFERENCES
-
- Lee T & Luo L Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

