COVID-19: Current Trends in Invitro Diagnostics
- PMID: 32641875
- PMCID: PMC7320251
- DOI: 10.1007/s12291-020-00906-5
COVID-19: Current Trends in Invitro Diagnostics
Abstract
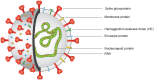
The novel coronavirus SARS-CoV-2 is the seventh known species of coronavirus, infectious to human beings. The pandemic COVID-19 spread all over the world with an unprecedented spreading rate after its first appearance in Wuhan, China. As a novel viral disease there in no antiviral treatment or vaccine for the COVID-19. At present, the early detection and the quarantine of infected patients are the ways to stop the spreading of the disease. This review will discuss about the current invitro diagnostic methods used worldwide for the early and accurate diagnosis of COVID-19. Currently the nucleic acid based polymerase chain reaction is used as the reliable diagnostic platform and antigen/antibody detection immunoassays are playing the role of screening tests for early detection and prognosis in COVID-19 treatment.
Keywords: CLIA; ELISA; Lateral flow immunoassay; Loop mediated isothermal amplification; Reverse transcriptase PCR; SARS-CoV-2.
© Association of Clinical Biochemists of India 2020.
Conflict of interest statement
Conflict of interestAll authors declare that there is no conflict of interest.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous