Practitioner Due Diligence: Real-World Lumacaftor/Ivacaftor Use
- PMID: 32641913
- PMCID: PMC7337128
- DOI: 10.5863/1551-6776-25.5.431
Practitioner Due Diligence: Real-World Lumacaftor/Ivacaftor Use
Abstract
Objective: Previous trials evaluated the efficacy of lumacaftor/ivacaftor in Phe508del homozygotes. These trials are limited by manufacturer sponsorship and were conducted under strict protocol. Additionally, this therapy is costly and does not allow for reduction in daily cystic fibrosis therapies. This study assessed the efficacy of lumacaftor/ivacaftor therapy and its effect on health care utilization in a real-world setting.
Methods: Retrospective chart review comparing the first 12 months of therapy to the 24 months prior was conducted to evaluate the impact of lumacaftor/ivacaftor on pulmonary function following a streamlined process for therapy introduction. The impact on body mass index and healthcare utilization were also evaluated. The following measurements were assessed: percent predicted forced expiratory volume in 1 second, body mass index and z-scores, number of admissions, length of stay, number of emergency department visits.
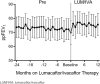
Results: Mean ppFEV1 was improved for the first 12 months on lumacaftor/ivacaftor treatment when compared with the 24 months prior: 78.8 (95% CI: 72.6, 84.9) vs 76.2 (95% CI: 70.1, 82.3) (p = 0.03). Body mass index significantly improved (patients ≥20 years), but improvement in BMI z-score (patients <20 years) was not significant. Number of admissions and LOS were significantly decreased, but ED visits were not.
Conclusions: Lumacaftor/ivacaftor is effective for improving ppFEV1 and BMI and for reducing health care utilization. However, this small reduction does not overcome the financial cost of treatment. Long-term outcomes and use must be studied to determine the overall effect of this therapy on cystic fibrosis interventions and their costs.
Keywords: cystic fibrosis; length of stay; lumacaftor, ivacaftor drug combination; patient admission.
Copyright Pediatric Pharmacy Association. All rights reserved. For permissions, email: mhelms@pediatricpharmacy.org 2020.
Conflict of interest statement
Disclosures Kelly Sakellaris reports personal fees from Abbvie Pharmaceuticals, outside the submitted work. Dr Stephan reports personal fees from Gilead Sciences, Inc, outside the submitted work. Karen McCoy reports grants from and attending investigator meetings for Vertex Pharmaceuticals, grants from Alcresta Pharmaceuticals, grants from Novartis Pharmaceuticals, grants from Savara Pharmaceuticals, grants from ProQR Pharmaceuticals, attending investigator meetings for Pharmaxis Pharmaceuticals, grants from Novoteris, grants from Nivalis, grants from and attending investigator meetings for Proteostasis Therapeutics, grants from Laurent Pharmaceuticals, grants from Translate Bio, grants from CFFT, grants from Corbus Pharmaceuticals, grants from Concert Pharmaceuticals, and grants from Gilead Pharmaceuticals, all outside the submitted work. Karen McCoy states that all the related funding is given to her institution of employment for the above-listed entities to support research trials. She received no direct money, and none of these relationships is in conflict with this manuscript. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- O'Sullivan AK, Sullivan J, Higuchi K, Montgomery AB. Health care utilization & costs for cystic fibrosis patients with pulmonary infections. Manag Care. 2011;20(2):37–44. - PubMed
-
- Pollack A. New York Times. New York, NY: 2015. Orkambi, a new cystic fibrosis drug, wins F.D.A. approval.https://www.nytimes.com/2015/07/03/business/orkambi-a-newcystic-fibrosis... Accessed September 19, 2018.
-
- Konstan MW, McKone EF, Moss RB et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508delCFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5(2):107–118. - PubMed
-
- Middleton EA, McCoy KS, Novak KJ et al. Practitioner due diligence real-world lumacaftor-ivacaftor use. Pediatric Pulmonol. 2017;52(S47):315–316.
LinkOut - more resources
Full Text Sources