Design and Synthesis of Novel Triazole-based Peptide Analogues as Anticancer Agents
- PMID: 32641940
- PMCID: PMC6934984
- DOI: 10.22037/ijpr.2019.111722.13320
Design and Synthesis of Novel Triazole-based Peptide Analogues as Anticancer Agents
Abstract
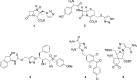
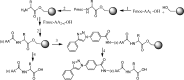
Cancer disease is a great concern in the worldwide public health and current treatments do not give satisfactory results, so, developing novel therapeutic agents to combat cancer is highly demanded. Nowadays, anticancer peptides (ACPs) are becoming promising anticancer drug candidates. This is due to several advantages inherited in peptide molecules, such as being usually with small size, high activity, low immunogenicity, good biocompatibility, diversity of sequence, and more modification sites for functionalization. To get benefit of these merits, in this work, we synthesized a new series of triazole- based analogues with peptide scaffold by employing click chemistry and evaluated their anticancer activities against breast, colon cancer cell lines as well as fibroblast cells using MTT assay. Our results suggest that peptide scaffolds containing 1H-1, 2, 3-triazole ring group are toxic against colon and breast cancer cells viability, and this effect was more pronounced on MDA-MB-231 cells compared with MCF-7 breast cells. As a conclusion, these designed peptide analogues may be good and safe candidates as future anticancer agents.
Keywords: Cancer; Click chemistry; MTT assay; Peptide analogues; Triazole rings.
Figures



References
-
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov. Today . 2010;15:40–56. - PubMed
-
- Merrifield RB. Solid phase peptide synthesis The synthesis of a tetrapeptide. J. Am. Chem. Soc. . 1963;85:2149–54.
-
- Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science . 1994;266:776–79. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
