Cellular metabolism and homeostasis in pluripotency regulation
- PMID: 32643102
- PMCID: PMC7452966
- DOI: 10.1007/s13238-020-00755-1
Cellular metabolism and homeostasis in pluripotency regulation
Abstract
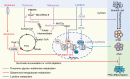
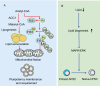
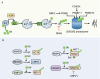
Pluripotent stem cells (PSCs) can immortally self-renew in culture with a high proliferation rate, and they possess unique metabolic characteristics that facilitate pluripotency regulation. Here, we review recent progress in understanding the mechanisms that link cellular metabolism and homeostasis to pluripotency regulation, with particular emphasis on pathways involving amino acid metabolism, lipid metabolism, the ubiquitin-proteasome system and autophagy. Metabolism of amino acids and lipids is tightly coupled to epigenetic modification, organelle remodeling and cell signaling pathways for pluripotency regulation. PSCs harness enhanced proteasome and autophagy activity to meet the material and energy requirements for cellular homeostasis. These regulatory events reflect a fine balance between the intrinsic cellular requirements and the extrinsic environment. A more complete understanding of this balance will pave new ways to manipulate PSC fate.
Keywords: amino acid metabolism; autophagy; lipid metabolism; pluripotent stem cell (PSC); ubiquitin-proteasome system (UPS).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

