COVID-19 Clinical Diagnostics and Testing Technology
- PMID: 32643218
- PMCID: PMC7361586
- DOI: 10.1002/phar.2439
COVID-19 Clinical Diagnostics and Testing Technology
Abstract
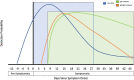
Given the global nature of the coronavirus disease 2019 (COVID-19) pandemic, the need for disease detection and expanding testing capacity remains critical priorities. This review discusses the technological advances in testing capability and methodology that are currently used or in development for detecting the novel coronavirus. We describe the current clinical diagnostics and technology, including molecular and serological testing approaches, for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) testing as well as address their advantages and limitations. Nucleic acid amplification technology for molecular diagnostics remains the gold standard for virus detection. We highlight alternative molecular detection techniques used for developing novel COVID-19 diagnostics on the horizon. Antibody response against SARS-CoV-2 remains poorly understood and proper validation of serology tests is necessary to demonstrate their accuracy and clinical utility. In order to bring the pandemic under control, we must speed up the development of rapid and widespread testing through improvements in clinical diagnostics and testing technology as well as access to these tools.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; antibody; antigen; serology.
© 2020 Pharmacotherapy Publications, Inc.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

