Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients
- PMID: 32643323
- PMCID: PMC7471041
- DOI: 10.1111/1759-7714.13541
Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients
Abstract
Background: Immune checkpoint inhibitors (ICIs) have significant clinical efficacy in the treatment of non-small cell lung cancer (NSCLC); however, the incidence of immune-related adverse events (irAEs) of up to 50% has prevented their widespread use. With the increase in the use of ICIs alone or as combination therapy, clinicians are required to have a better understanding of irAEs and be able to manage them systematically. In this study, we aimed to assess the incidence of irAEs associated with ICIs.
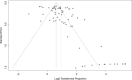
Methods: We searched PubMed, Embase, and the Web of Science databases, and also included relevant literature references to widen our search. The relevant data with inclusion criteria were performed using RevMan 3.6.0 for meta-analysis. We undertook a systematic literature search which included published data up to December 2019.
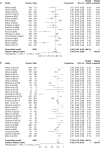
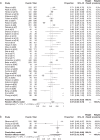
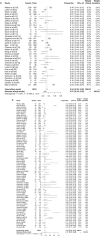
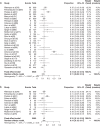
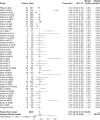
Results: Overall, 147 articles and 23 761 cancer patients with 11 different ICI treatment-related (grade 1-5 and 3-5) irAEs were included in the study. There were 46 articles on pembrolizumab (6598 patients), 27 on nivolumab (3576 patients), 13 on atezolizumab (2787 patients), 12 on avelumab (3213 patients), 10 on durvalumab (1780 patients), 22 on ipilimumab (4067 patients), eight on tremelimumab (1158 patients), three on JS001 (223 patients), four on camrelizumab (SHR-1210) (178 patients), one on sintilimab (96 patients), and one on cemiplimab (85 patients). Grade 1-5 irAEs were: cytotoxic T lymphocyte antigen 4 (CTLA-4) (82.87%), programmed cell death 1 (PD-1) (71.89%), and programmed cell death ligand-1 (PD-L1) (58.95%). Subgroup analysis was: Avelumab (44.53%), durvalumab (66.63%), pembrolizumab (67.25%), atezolizumab (68.77%), nivolumab (76.25%), Ipilimumab (82.18%), and tremelimumab (86.78%). Grade 3-5 irAEs were: CTLA-4 (27.22%), PD-1(17.29%), and PD-L1(17.29%). Subgroup analysis was: Avelumab (5.86%), durvalumab (13.43%), atezolizumab (14.45%), nivolumab (15.72%), pembrolizumab (16.58%), tremelimumab (22.04%), and ipilimumab (28.27%).
Conclusions: This meta-analysis confirmed that anti-PD-1 and anti-PD-L1 inhibitors had a lower incidence of irAEs compared with anti-CTLA-4 inhibitors.
Keywords: Cancer; immune checkpoint inhibitor (ICI); immune-related adverse events (irAEs).
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures









References
-
- Naoya Y, Tatsuya T, Manabu F, et al Phase 1b study of pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE‐041). Cancer Chemother Pharmacol 2017; 79: 651–660. https://doi:10.1007/s00280-016-3237-x. - DOI - PMC - PubMed
-
- Ding W, LaPlant BR, Call TG Ding W et al Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017; 129 26:3419–3427. https://doi:10.1182/blood-2017-02-765685. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

