Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment
- PMID: 32643603
- PMCID: PMC7284240
- DOI: 10.1016/j.cell.2020.06.010
Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment
Abstract
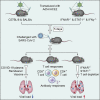
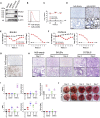
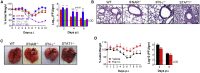
COVID-19, caused by SARS-CoV-2, is a virulent pneumonia, with >4,000,000 confirmed cases worldwide and >290,000 deaths as of May 15, 2020. It is critical that vaccines and therapeutics be developed very rapidly. Mice, the ideal animal for assessing such interventions, are resistant to SARS-CoV-2. Here, we overcome this difficulty by exogenous delivery of human ACE2 with a replication-deficient adenovirus (Ad5-hACE2). Ad5-hACE2-sensitized mice developed pneumonia characterized by weight loss, severe pulmonary pathology, and high-titer virus replication in lungs. Type I interferon, T cells, and, most importantly, signal transducer and activator of transcription 1 (STAT1) are critical for virus clearance and disease resolution in these mice. Ad5-hACE2-transduced mice enabled rapid assessments of a vaccine candidate, of human convalescent plasma, and of two antiviral therapies (poly I:C and remdesivir). In summary, we describe a murine model of broad and immediate utility to investigate COVID-19 pathogenesis and to evaluate new therapies and vaccines.
Keywords: COVID-19; SARS-CoV-2; mouse model; pathogenesis; therapeutics; vaccine.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



References
-
- Anderson R.D., Haskell R.E., Xia H., Roessler B.J., Davidson B.L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. - PubMed
-
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;2020:7. - PubMed
-
- Bennett N.T., Schultz G.S. Growth factors and wound healing: Part II. Role in normal and chronic wound healing. Am. J. Surg. 1993;166:74–81. - PubMed
-
- Boudewijns R., Thibaut H.J., Kaptein S.J.F., Li R., Vergote V., Seldeslachts L., De Keyzer C., Sharma S., Jansen S., Weyenbergh J.V. STAT2 signaling as double-edged sword restricting viral dissemination but driving severe pneumonia in SARS-CoV-2 infected hamsters. bioRxiv. 2020 doi: 10.1101/2020.04.23.056838. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

