Disease Course in Patients With Pentosan Polysulfate Sodium-Associated Maculopathy After Drug Cessation
- PMID: 32644147
- PMCID: PMC7349079
- DOI: 10.1001/jamaophthalmol.2020.2349
Disease Course in Patients With Pentosan Polysulfate Sodium-Associated Maculopathy After Drug Cessation
Abstract
Importance: Recent studies have linked a vision-threatening maculopathy with long-term use of pentosan polysulfate sodium (PPS).
Objective: To evaluate the disease course in PPS-associated maculopathy after drug cessation.
Design, setting, and participants: In this retrospective case series, patients diagnosed with PPS-associated maculopathy with at least 6 months of follow-up after drug cessation who were treated at the Emory Eye Center, Atlanta, Georgia, or the Casey Eye Institute, Portland, Oregon, were included. Data were collected from April 2014 through November 2019.
Main outcomes and measures: Change in visual acuity and retinal imaging characteristics over time.
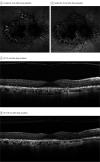
Results: Of the 11 included patients, all were female, and the median (interquartile range [IQR]) age was 53 (44-63) years. Participants had a baseline visit at a median (IQR) of 2 (0-4) months after drug cessation and were subsequently observed for a median (IQR) of 12 (8-26) months. The median (IQR) cumulative PPS exposure was 1.97 (1.55-2.18) kg. No eyes exhibited a demonstrable improvement in disease after discontinuing PPS. A total of 9 of 11 patients (82%) reported worsening visual symptoms at the final visit. The mean (SD) logMAR visual acuity was 0.14 (0.23) and 0.14 (0.34) at the baseline and final visit, respectively. Visual acuity improved by 2 or more Snellen lines in 1 eye (5%) and declined by 2 or more Snellen lines in 2 eyes of 1 patient (9%). There was evolution in the pattern of fundus autofluorescence changes and/or optical coherence tomography findings in all eyes. A total of 17 eyes (77%) exhibited expansion of the area of involved tissue. A total of 7 eyes (32%) had macular retinal pigment epithelium atrophy at the baseline visit, and atrophy enlarged after discontinuation of PPS in all 7 eyes, with a median (IQR) growth rate of 0.32 (0.13-0.38) mm per year.
Conclusions and relevance: These retrospective data among 11 patients suggest PPS-associated maculopathy continues to evolve after drug cessation for at least 10 years. In some cases, progressive retinal pigment epithelium atrophy encroaches on the foveal center and thus may pose a long-term threat to central vision.
Conflict of interest statement
Figures


Comment in
-
Pentosan Polysulfate Maculopathy-Prescribers Should Be Aware.JAMA Ophthalmol. 2020 Aug 1;138(8):900-902. doi: 10.1001/jamaophthalmol.2020.2364. JAMA Ophthalmol. 2020. PMID: 32644128 No abstract available.
-
Long-term Macular Effects of Pentosan Polysulfate Sodium Exposure Uncertain-Let's Not Jump to Conclusions.JAMA Ophthalmol. 2021 Mar 1;139(3):364. doi: 10.1001/jamaophthalmol.2020.6615. JAMA Ophthalmol. 2021. PMID: 33538763 No abstract available.
-
Long-term Macular Effects of Pentosan Polysulfate Sodium Exposure Uncertain-Let's Not Jump to Conclusions-Reply.JAMA Ophthalmol. 2021 Mar 1;139(3):364-365. doi: 10.1001/jamaophthalmol.2020.6618. JAMA Ophthalmol. 2021. PMID: 33538767 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

