Switches of SOX17 and SOX2 expression in the development of squamous metaplasia and squamous intraepithelial lesions of the uterine cervix
- PMID: 32644288
- PMCID: PMC7476841
- DOI: 10.1002/cam4.3201
Switches of SOX17 and SOX2 expression in the development of squamous metaplasia and squamous intraepithelial lesions of the uterine cervix
Abstract
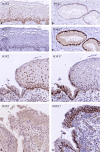
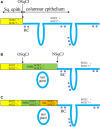
Aims: The dynamics and topographical distribution of SOX17 and SOX2 expression was studied in the transformation zone (TZ) of the uterine cervix. This TZ is a dynamic area where switches from glandular into squamous epithelium can be recognized, new squamocolumnar junctions are formed, and premalignant lesions originate. SOX17 and SOX2 show mutually exclusive expression patterns in the normal uterine cervix, with SOX2 being exclusively found in squamous epithelium, while SOX17 is detected in endocervical columnar cells and reserve cells.
Methods and results: Normal cervices and squamous intraepithelial lesions (SIL) were studied with immunohistochemistry, methylation of SOX17, human papilloma virus (HPV) genotyping, and in situ hybridization. In the TZ squamous metaplasia originating from these reserve cells can still show SOX17 expression, while also remnants of SOX17-positive immature metaplasia can be recognized in the normal squamous epithelium. SOX17 expression is gradually lost during maturation, resulting in the exclusive expression of SOX2 in the majority of (SIL). This loss of SOX17 expression is independent of methylation of the CpG island in its promotor region. HPV can be detected in SOX17-positive immature metaplastic regions in the immediate vicinity of SOX2-positive SIL, suggesting that switches in SOX17 and 2 expression can occur upon HPV infection.
Conclusions: This switch in expression, and the strong association between the distribution of reserve cells and squamous areas within the columnar epithelium in the TZ, suggests that reserve cell proliferations, next to basal cells in the squamous epithelium, are potential targets for the formation of squamous lesions upon viral infection.
Keywords: SOX17; SOX2; cervical preneoplasia; keratins; reserve cells; squamocolumnar junction; squamous intraepithelial lesions; transformation zone.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors are responsible for disclosing all financial and personal relationships between themselves and others that might bias their work. There are no potential conflicts.
Figures




References
-
- Wright TC, Ferenczy A. Benign diseases of the cervix In: Kurman RJ, ed. Blaustein's Pathology of the Female Genital Tract. New York: Springer; 1994;203‐227.
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890‐907. - PubMed
-
- Herfs M, Hubert P, Delvenne P. Epithelial metaplasia: adult stem cell reprogramming and (pre)neoplastic transformation mediated by inflammation? Trends Mol Med. 2009;15:245‐253. - PubMed
-
- Herfs M, Hubert P, Moutschen M, Delvenne P. Mucosal junctions: open doors to HPV and HIV infections? Trends Microbiol. 2011;19:114‐120. - PubMed

