Edward F. Adolph Distinguished Lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome
- PMID: 32644910
- PMCID: PMC7473948
- DOI: 10.1152/japplphysiol.00381.2020
Edward F. Adolph Distinguished Lecture. Skeletal muscle atrophy: Multiple pathways leading to a common outcome
Abstract
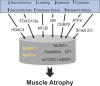
Skeletal muscle atrophy continues to be a serious consequence of many diseases and conditions for which there is no treatment. Our understanding of the mechanisms regulating skeletal muscle mass has improved considerably over the past two decades. For many years it was known that skeletal muscle atrophy resulted from an imbalance between protein synthesis and protein breakdown, with the net balance shifting toward protein breakdown. However, the molecular and cellular mechanisms underlying the increased breakdown of myofibrils was unknown. Over the past two decades, numerous reports have identified novel genes and signaling pathways that are upregulated and activated in response to stimuli such as disuse, inflammation, metabolic stress, starvation and others that induce muscle atrophy. This review summarizes the discovery efforts performed in the identification of several pathways involved in the regulation of skeletal muscle mass: the mammalian target of rapamycin (mTORC1) and the ubiquitin proteasome pathway and the E3 ligases, MuRF1 and MAFbx. While muscle atrophy is a common outcome of many diseases, it is doubtful that a single gene or pathway initiates or mediates the breakdown of myofibrils. Interestingly, however, is the observation that upregulation of the E3 ligases, MuRF1 and MAFbx, is a common feature of many divergent atrophy conditions. The challenge for the field of muscle biology is to understand how all of the various molecules, transcription factors, and signaling pathways interact to produce muscle atrophy and to identify the critical factors for intervention.
Keywords: MAFbx; MuRF1; mTORC1; protein synthesis; ubiquitin proteasome pathway.
Conflict of interest statement
Dr. Bodine holds equity in Emmyon, Inc., and serves on the Scientific Advisory Board.
Figures



References
-
- Arnold J, Campbell IT, Samuels TA, Devlin JC, Green CJ, Hipkin LJ, MacDonald IA, Scrimgeour CM, Smith K, Rennie MJ. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci (Lond) 84: 655–661, 1993. doi: 10.1042/cs0840655. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

