Cancer cells educate natural killer cells to a metastasis-promoting cell state
- PMID: 32645139
- PMCID: PMC7480097
- DOI: 10.1083/jcb.202001134
Cancer cells educate natural killer cells to a metastasis-promoting cell state
Abstract
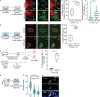
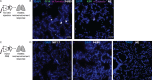
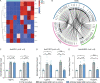
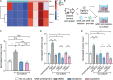
Natural killer (NK) cells have potent antitumor and antimetastatic activity. It is incompletely understood how cancer cells escape NK cell surveillance. Using ex vivo and in vivo models of metastasis, we establish that keratin-14+ breast cancer cells are vulnerable to NK cells. We then discovered that exposure to cancer cells causes NK cells to lose their cytotoxic ability and promote metastatic outgrowth. Gene expression comparisons revealed that healthy NK cells have an active NK cell molecular phenotype, whereas tumor-exposed (teNK) cells resemble resting NK cells. Receptor-ligand analysis between teNK cells and tumor cells revealed multiple potential targets. We next showed that treatment with antibodies targeting TIGIT, antibodies targeting KLRG1, or small-molecule inhibitors of DNA methyltransferases (DMNT) each reduced colony formation. Combinations of DNMT inhibitors with anti-TIGIT or anti-KLRG1 antibodies further reduced metastatic potential. We propose that NK-directed therapies targeting these pathways would be effective in the adjuvant setting to prevent metastatic recurrence.
© 2020 Chan et al.
Figures






References
-
- Chen Z., Quan L., Huang A., Zhao Q., Yuan Y., Yuan X., Shen Q., Shang J., Ben Y., Qin F.X., et al. . 2018. seq-ImmuCC: Cell-Centric View of Tissue Transcriptome Measuring Cellular Compositions of Immune Microenvironment From Mouse RNA-Seq Data. Front. Immunol. 9:1286 10.3389/fimmu.2018.01286 - DOI - PMC - PubMed
-
- Cherfils-Vicini J., Iltis C., Cervera L., Pisano S., Croce O., Sadouni N., Győrffy B., Collet R., Renault V.M., Rey-Millet M., et al. . 2019. Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF2. EMBO J. 38 e100012 10.15252/embj.2018100012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

