Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study
- PMID: 32645347
- PMCID: PMC7336131
- DOI: 10.1016/S0140-6736(20)31483-5
Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study
Abstract
Background: Spain is one of the European countries most affected by the COVID-19 pandemic. Serological surveys are a valuable tool to assess the extent of the epidemic, given the existence of asymptomatic cases and little access to diagnostic tests. This nationwide population-based study aims to estimate the seroprevalence of SARS-CoV-2 infection in Spain at national and regional level.
Methods: 35 883 households were selected from municipal rolls using two-stage random sampling stratified by province and municipality size, with all residents invited to participate. From April 27 to May 11, 2020, 61 075 participants (75·1% of all contacted individuals within selected households) answered a questionnaire on history of symptoms compatible with COVID-19 and risk factors, received a point-of-care antibody test, and, if agreed, donated a blood sample for additional testing with a chemiluminescent microparticle immunoassay. Prevalences of IgG antibodies were adjusted using sampling weights and post-stratification to allow for differences in non-response rates based on age group, sex, and census-tract income. Using results for both tests, we calculated a seroprevalence range maximising either specificity (positive for both tests) or sensitivity (positive for either test).
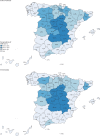
Findings: Seroprevalence was 5·0% (95% CI 4·7-5·4) by the point-of-care test and 4·6% (4·3-5·0) by immunoassay, with a specificity-sensitivity range of 3·7% (3·3-4·0; both tests positive) to 6·2% (5·8-6·6; either test positive), with no differences by sex and lower seroprevalence in children younger than 10 years (<3·1% by the point-of-care test). There was substantial geographical variability, with higher prevalence around Madrid (>10%) and lower in coastal areas (<3%). Seroprevalence among 195 participants with positive PCR more than 14 days before the study visit ranged from 87·6% (81·1-92·1; both tests positive) to 91·8% (86·3-95·3; either test positive). In 7273 individuals with anosmia or at least three symptoms, seroprevalence ranged from 15·3% (13·8-16·8) to 19·3% (17·7-21·0). Around a third of seropositive participants were asymptomatic, ranging from 21·9% (19·1-24·9) to 35·8% (33·1-38·5). Only 19·5% (16·3-23·2) of symptomatic participants who were seropositive by both the point-of-care test and immunoassay reported a previous PCR test.
Interpretation: The majority of the Spanish population is seronegative to SARS-CoV-2 infection, even in hotspot areas. Most PCR-confirmed cases have detectable antibodies, but a substantial proportion of people with symptoms compatible with COVID-19 did not have a PCR test and at least a third of infections determined by serology were asymptomatic. These results emphasise the need for maintaining public health measures to avoid a new epidemic wave.
Funding: Spanish Ministry of Health, Institute of Health Carlos III, and Spanish National Health System.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
SARS-CoV-2 seroprevalence in COVID-19 hotspots.Lancet. 2020 Aug 22;396(10250):514-515. doi: 10.1016/S0140-6736(20)31482-3. Epub 2020 Jul 6. Lancet. 2020. PMID: 32645348 Free PMC article. No abstract available.
-
SARS-CoV-2 seroprevalence in Spain.Lancet. 2020 Nov 7;396(10261):1484. doi: 10.1016/S0140-6736(20)32273-X. Lancet. 2020. PMID: 33160560 Free PMC article. No abstract available.
-
SARS-CoV-2 seroprevalence in Spain - Authors' reply.Lancet. 2020 Nov 7;396(10261):1484-1485. doi: 10.1016/S0140-6736(20)32266-2. Lancet. 2020. PMID: 33160561 Free PMC article. No abstract available.
-
SARS-CoV-2 seroprevalence in Spain.Lancet. 2020 Nov 7;396(10261):1484. doi: 10.1016/S0140-6736(20)32272-8. Lancet. 2020. PMID: 33160562 Free PMC article. No abstract available.
-
[Comparative study of the COVID-19 admissions between first and second wave in a cohort of 1,235 patients].Rev Esp Quimioter. 2021 Aug;34(4):387-389. doi: 10.37201/req/005.2021. Epub 2021 Apr 29. Rev Esp Quimioter. 2021. PMID: 33913313 Free PMC article. Spanish. No abstract available.
References
-
- Instituto de Salud Carlos III COVID-19 in Spain. https://cnecovid.isciii.es/covid19
-
- European Centre for Disease Prevention and Control COVID-19 situation update worldwide, as of 18 June 2020. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

