Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling
- PMID: 32645833
- PMCID: PMC7407639
- DOI: 10.3390/ph13070144
Loss of miR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling
Abstract
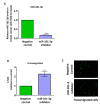
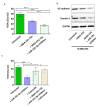
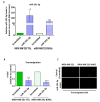
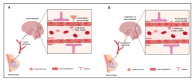
Brain metastases represent one of the incurable end stages in breast cancer (BC). Developing effective or preventive treatments is hampered by a lack of knowledge on the molecular mechanisms driving brain metastasis. Transmigration of BC cells through the brain endothelium is a key event in the pathogenesis of brain metastasis. In this study, we identified miR-101-3p as a critical micro-RNA able to reduce transmigration of BC cells through the brain endothelium. Our results revealed that miR-101-3p expression is downregulated in brain metastatic BC cells compared to less invasive variants, and varies inversely compared to the brain metastatic propensity of BC cells. Using a loss-and-gain of function approach, we found that miR-101-3p downregulation increased transmigration of BC cells through the brain endothelium in vitro by inducing COX-2 expression in cancer cells, whereas ectopic restoration of miR-101-3p exerted a metastasis-reducing effect. In regulatory experiments, we found that miR-101-3p mediated its effect by modulating COX-2-MMP1 signaling capable of degrading the inter-endothelial junctions (claudin-5 and VE-cadherin), key components of the brain endothelium. These findings suggest that miR-101-3p plays a critical role in the transmigration of breast cancer cells through the brain endothelium by modulating the COX-2-MMP1 signaling and thus may serve as a therapeutic target that can be exploited to prevent or suppress brain metastasis in human breast cancer.
Keywords: blood–brain barrier; brain metastasis; breast cancer; micro-RNA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Weidle U.H., Niewöhner J., Tiefenthaler G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genom. Proteom. 2015;12:167–177. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

