Polyphenols in Dental Applications
- PMID: 32645860
- PMCID: PMC7552636
- DOI: 10.3390/bioengineering7030072
Polyphenols in Dental Applications
Abstract
(1) Background: polyphenols are a broad class of molecules extracted from plants and have a large repertoire of biological activities. Biomimetic inspiration from the effects of tea or red wine on the surface of cups or glass lead to the emergence of versatile surface chemistry with polyphenols. Owing to their hydrogen bonding abilities, coordination chemistry with metallic cations and redox properties, polyphenols are able to interact, covalently or not, with a large repertoire of chemical moieties, and can hence be used to modify the surface chemistry of almost all classes of materials. (2) Methods: the use of polyphenols to modify the surface properties of dental materials, mostly enamel and dentin, to afford them with better adhesion to resins and improved biological properties, such as antimicrobial activity, started more than 20 years ago, but no general overview has been written to our knowledge. (3) Results: the present review is aimed to show that molecules from all the major classes of polyphenolics allow for low coast improvements of dental materials and engineering of dental tissues.
Keywords: antibacterial activity; dental resins; dentin; enamel; interactions with collagen; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
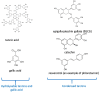






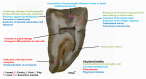
References
-
- Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64(Suppl. 3):S112–S120. - PubMed
-
- Handique J.G., Baruah J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002;52:163–188. doi: 10.1016/S1381-5148(02)00091-3. - DOI
-
- Sánchez M.C., Ribeiro-Vidal H., Esteban-Fernández A., Bartolomé B., Figuero E., Moreno-Arribas M.V., Sanz M., Herrera D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019;19:145. doi: 10.1186/s12906-019-2533-5. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

