Prospective Assessment of Systemic MicroRNAs as Markers of Response to Neoadjuvant Chemotherapy in Breast Cancer
- PMID: 32645898
- PMCID: PMC7408914
- DOI: 10.3390/cancers12071820
Prospective Assessment of Systemic MicroRNAs as Markers of Response to Neoadjuvant Chemotherapy in Breast Cancer
Abstract
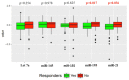
Neoadjuvant chemotherapy (NACT) is used in locally advanced breast cancer to reduce tumour burden prior to surgical resection. However, only a subset of NACT treated patients will respond to treatment or achieve a pathologic complete response (pCR). This multicenter, prospective study (CTRIAL-IE (ICORG) 10-11 study) evaluated circulating microRNA as novel non-invasive prognostic biomarkers of NACT response in breast cancer. Selected circulating microRNAs (Let-7a, miR-21, miR-145, miR-155, miR-195) were quantified from patients undergoing standard of care NACT treatment (n = 114) from whole blood at collected at diagnosis, and the association with NACT response and clinicopathological features evaluated. NACT responders had significantly lower levels of miR-21 (p = 0.036) and miR-195 (p = 0.017), compared to non-responders. Evaluating all breast cancer cases miR-21 was found to be an independent predictor of response (OR 0.538, 95% CI 0.308-0.943, p < 0.05). Luminal cancer NACT responders were found to have significantly decreased levels of miR-145 (p = 0.033) and miR-21 (p = 0.048), compared to non-responders. This study demonstrates the prognostic ability of miR-21, miR-195 and miR-145 as circulating biomarkers stratifying breast cancer patients by NACT response, identifying patients that will derive the maximum benefit from chemotherapy.
Keywords: biomarker; breast; chemotherapy; microRNA; neoadjuvant; prognostic.
Conflict of interest statement
M. Kerin, H. Heneghan and N. Miller: circulating miR-195 as a biomarker patent. All miRNA were measured on blinded samples and the unblinded analysis was performed by independent study statisticians. All other authors declare no potential conflicts of interest.
Figures



References
-
- Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., Bonnefoi H., Cameron D., Gianni L., Valagussa P., et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. - DOI - PubMed
-
- Battisti N.M.L., True V., Chaabouni N., Chopra N., Lee K., Shepherd S., Shapira-Rotenberg T., Joshi R., McGrath S., Okines A., et al. Pathological complete response to neoadjuvant systemic therapy in 789 early and locally advanced breast cancer patients: The Royal Marsden experience. Breast Cancer Res. Treat. 2019;179:101–111. doi: 10.1007/s10549-019-05444-0. - DOI - PubMed
-
- Fayanju O.M., Ren Y., Thomas S.M., Greenup R.A., Plichta J.K., Rosenberger L.H., Tamirisa N., Force J., Boughey J.C., Hyslop T., et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB) Ann. Surg. 2018;268:591–601. doi: 10.1097/SLA.0000000000002953. - DOI - PMC - PubMed
-
- Goldhirsch A., Wood W.C., Coates A.S., Gelber R.D., Thürlimann B., Senn H.-J., Members P. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

