The Effect of Ethylene on the Color Change and Resistance to Botrytis cinerea Infection in 'Kyoho' Grape Fruits
- PMID: 32645910
- PMCID: PMC7404975
- DOI: 10.3390/foods9070892
The Effect of Ethylene on the Color Change and Resistance to Botrytis cinerea Infection in 'Kyoho' Grape Fruits
Abstract
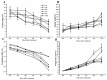
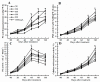
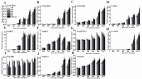
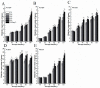
The formation of grape quality and the mechanism of resistance against foreign pathogens affect the storage stability of fruits during post-harvest handling. Ethylene plays a crucial role in regulating the ripeness of fruits and can be used as an exogenous regulator to resist exogenous pathogens. In this study, we used different concentrations of ethephon for treatment of grape fruits before veraison, analyzed the anthocyanin content, soluble solids, titratable acid, and determined fruit firmness and cell wall metabolism-related enzymes during fruit development. Results showed that exogenous ethephon promoted the early coloration of grape fruits and increased the coloring-related genes myeloblastosis A1(MYBA1), myeloblastosis A2(MYBA2), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3'-hydroxylase gene (F3'H), flavonoid 3', 5'hydroxylase (F3'5'H), 3-O-flavonoid glucosyltransferase (UFGT), and glutathione S-transferase (GST), softening related genes Polygalacturonase(PG), pectinate lyases(PL) and Pectin methylesterase( PME, as well as ethylene metabolism pathway-related genes 1-aminocyclopropane-1-carboxylic acid synthase 1(ACS1), 1-aminocyclopropane-1-carboxylic acid oxidase 2 (ACO2), ethylene receptor gene(ETR2), and ethylene-insensitive 3 (EIN3). Ethephon treatment also increased soluble solids and decreased titratable acid in grape fruit. Fruits pretreated with ethephon were inoculated with Botrytis cinerea, which led to resistance in grape fruit through activation of the antioxidant system. The expression levels of disease resistance-related genes including VvPAD4, VvPIP1, VvNAC26, VvDREB, VvAPX, Vvpgip, VvWRKY70, VvMYC2, VvNPR1 also increased in inoculated fruit with pathogen following ethephon pretreatment. Furthermore, we monitored ethylene response factor 1(ERF1) transcription factor, which could interact with protein EIN3 during ethylene signal transduction and mediate fruit resistance against B. cinerea infection. Meanwhile, overexpression of VvERF1 vectorin strawberry fruits reduced the susceptibility to B. cinerea infection. We suggest that ethylene can induce resistance in ripened fruits after B. cinerea infection and provide adequate postharvest care.
Keywords: Botrytis cinerea; VvERF1; ethephon; gene expression; grape.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Cappellini R.A., Ceponis M.J., Lightner G.W. Disorders in table grape shipments to the New-York market, 1972–1984. Plant Dis. 1986;70:1075–1079. doi: 10.1094/PD-70-1075. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

