Synthesis of Ordered Mesoporous Zr-Al Composite Oxides with Excellent Structural and Textural Properties and Extremely High Stability
- PMID: 32645947
- PMCID: PMC7372406
- DOI: 10.3390/ma13133036
Synthesis of Ordered Mesoporous Zr-Al Composite Oxides with Excellent Structural and Textural Properties and Extremely High Stability
Abstract
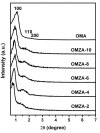
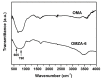
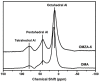
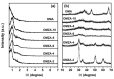
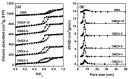
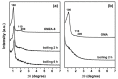
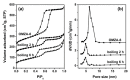
Ordered mesoporous Zr-Al composite oxide materials (denoted as OMZA-x) with different Zr contents have been synthesized by a solvent evaporation-inducing self-assembly procedure associated with a thermal treatment at 100 °C. A cooperative co-assembly process of amphiphilic triblock copolymer F127 molecules and inorganic hydroxyl species originated from the hydrolysis of Zr and Al precursors was proposed to explain the synthesis of OMZA-x. Compared to ordered mesoporous alumina prepared without introducing Zr species, the resultant OMZA-x exhibited a much more ordered mesostructure combined with a distinct increase in the pore volume and specific surface area. The highly homogenous doping of Zr into the mesopore walls together with the formation of Zr-O-Al bonds can effectively enhance the thermal and hydrothermal stability of OMZA-x. For instance, the ordered mesostructure and excellent textural properties of OMZA-6 prepared with the optimum atomic ratio of Al to Zr of 6 could be well maintained even after a high-temperature treatment at 1000 °C for 1 h or a hydrothermal treatment at 100 °C for 6 h.
Keywords: Zr-Al composite oxide; cooperative co-assembly; hydrothermal stability; mesoporous materials; thermal stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Facile synthesis and unique physicochemical properties of three-dimensionally ordered macroporous magnesium oxide, gamma-alumina, and ceria-zirconia solid solutions with crystalline mesoporous walls.Inorg Chem. 2009 May 18;48(10):4421-34. doi: 10.1021/ic900132k. Inorg Chem. 2009. PMID: 19348445
-
sp2-Hybridized Carbon-Containing Block Copolymer Templated Synthesis of Mesoporous Semiconducting Metal Oxides with Excellent Gas Sensing Property.Acc Chem Res. 2019 Mar 19;52(3):714-725. doi: 10.1021/acs.accounts.8b00598. Epub 2019 Mar 4. Acc Chem Res. 2019. PMID: 30829473
-
Evaporation-induced self-assembly synthesis of nanostructured alumina-based mixed metal oxides with tailored porosity.J Colloid Interface Sci. 2019 Mar 1;537:725-735. doi: 10.1016/j.jcis.2018.11.044. Epub 2018 Nov 14. J Colloid Interface Sci. 2019. PMID: 30470518
-
A vesicle-aggregation-assembly approach to highly ordered mesoporous γ-alumina microspheres with shifted double-diamond networks.Chem Sci. 2018 Aug 17;9(39):7705-7714. doi: 10.1039/c8sc02967a. eCollection 2018 Oct 21. Chem Sci. 2018. PMID: 30393532 Free PMC article.
-
Smart synthesis of highly porous metal oxide powders with the self-assembly of amphiphilic organic compounds.Dalton Trans. 2024 Jul 30;53(30):12434-12441. doi: 10.1039/d4dt01427h. Dalton Trans. 2024. PMID: 38922321 Review.
References
-
- Beck J.S., Vartuli J.C., Roth W.J., Leonowicz M.E., Kresge C.T., Schmitt K.D., Chu C.T., Olson D.H., Sheppard E.W., Mccullen S.B. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114:10834–10843. doi: 10.1021/ja00053a020. - DOI
-
- Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi: 10.1038/359710a0. - DOI
-
- And M.K., Jaroniec M., And R.R., Joo S.H. Characterization of Ordered Mesoporous Carbons Synthesized Using MCM-48 Silicas as Templates. J. Phys. Chem. B. 2000;104:7960–7968. doi: 10.1021/jp000861u. - DOI
Grants and funding
- 21975174/the National Natural Science Foundation of China
- 21878203/the National Natural Science Foundation of China
- 21706176/the National Natural Science Foundation of China
- 201801D121057/the Applied Basic Research Foundation of Shanxi Province
- 201801D121061/the Applied Basic Research Foundation of Shanxi Province
LinkOut - more resources
Full Text Sources

