Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts
- PMID: 32645959
- PMCID: PMC7408991
- DOI: 10.3390/cancers12071822
Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts
Abstract
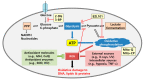
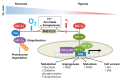
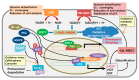
Modest levels of reactive oxygen species (ROS) are necessary for intracellular signaling, cell division, and enzyme activation. These ROS are later eliminated by the body's antioxidant defense system. High amounts of ROS cause carcinogenesis by altering the signaling pathways associated with metabolism, proliferation, metastasis, and cell survival. Cancer cells exhibit enhanced ATP production and high ROS levels, which allow them to maintain elevated proliferation through metabolic reprograming. In order to prevent further ROS generation, cancer cells rely on more glycolysis to produce ATP and on the pentose phosphate pathway to provide NADPH. Pro-oxidant therapy can induce more ROS generation beyond the physiologic thresholds in cancer cells. Alternatively, antioxidant therapy can protect normal cells by activating cell survival signaling cascades, such as the nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1) pathway, in response to radio- and chemotherapeutic drugs. Nrf2 is a key regulator that protects cells from oxidative stress. Under normal conditions, Nrf2 is tightly bound to Keap1 and is ubiquitinated and degraded by the proteasome. However, under oxidative stress, or when treated with Nrf2 activators, Nrf2 is liberated from the Nrf2-Keap1 complex, translocated into the nucleus, and bound to the antioxidant response element in association with other factors. This cascade results in the expression of detoxifying enzymes, including NADH-quinone oxidoreductase 1 (NQO1) and heme oxygenase 1. NQO1 and cytochrome b5 reductase can neutralize ROS in the plasma membrane and induce a high NAD+/NADH ratio, which then activates SIRT1 and mitochondrial bioenergetics. NQO1 can also stabilize the tumor suppressor p53. Given their roles in cancer pathogenesis, redox homeostasis and the metabolic shift from glycolysis to oxidative phosphorylation (through activation of Nrf2 and NQO1) seem to be good targets for cancer therapy. Therefore, Nrf2 modulation and NQO1 stimulation could be important therapeutic targets for cancer prevention and treatment.
Keywords: NQO1; Nrf2-Keap1; cancer; glycolysis; oxidative phosphorylation; oxidative stress.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

