Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis
- PMID: 32645995
- PMCID: PMC7400846
- DOI: 10.3390/nu12072021
Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis
Abstract
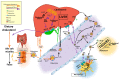
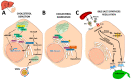
Cholesterol, the most important sterol in mammals, helps maintain plasma membrane fluidity and is a precursor of bile acids, oxysterols, and steroid hormones. Cholesterol in the body is obtained from the diet or can be de novo synthetized. Cholesterol homeostasis is mainly regulated by the liver, where cholesterol is packed in lipoproteins for transport through a tightly regulated process. Changes in circulating lipoprotein cholesterol levels lead to atherosclerosis development, which is initiated by an accumulation of modified lipoproteins in the subendothelial space; this induces significant changes in immune cell differentiation and function. Beyond lesions, cholesterol levels also play important roles in immune cells such as monocyte priming, neutrophil activation, hematopoietic stem cell mobilization, and enhanced T cell production. In addition, changes in cholesterol intracellular metabolic enzymes or transporters in immune cells affect their signaling and phenotype differentiation, which can impact on atherosclerosis development. In this review, we describe the main regulatory pathways and mechanisms of cholesterol metabolism and how these affect immune cell generation, proliferation, activation, and signaling in the context of atherosclerosis.
Keywords: atherosclerosis; cholesterol; hematopoiesis; immune cells; inflammation; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

