The cytoskeletal regulator HEM1 governs B cell development and prevents autoimmunity
- PMID: 32646852
- PMCID: PMC7116756
- DOI: 10.1126/sciimmunol.abc3979
The cytoskeletal regulator HEM1 governs B cell development and prevents autoimmunity
Abstract
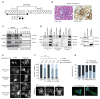
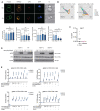
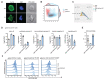
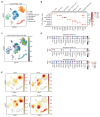
The WAVE regulatory complex (WRC) is crucial for assembly of the peripheral branched actin network constituting one of the main drivers of eukaryotic cell migration. Here, we uncover an essential role of the hematopoietic-specific WRC component HEM1 for immune cell development. Germline-encoded HEM1 deficiency underlies an inborn error of immunity with systemic autoimmunity, at cellular level marked by WRC destabilization, reduced filamentous actin, and failure to assemble lamellipodia. Hem1-/- mice display systemic autoimmunity, phenocopying the human disease. In the absence of Hem1, B cells become deprived of extracellular stimuli necessary to maintain the strength of B cell receptor signaling at a level permissive for survival of non-autoreactive B cells. This shifts the balance of B cell fate choices toward autoreactive B cells and thus autoimmunity.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Suetsugu S, Miki H, Takenawa T. Identification of Two Human WAVE/SCAR Homologues as General Actin Regulatory Molecules Which Associate with the Arp2/3 Complex. Biochem Biophys Research Commun. 1999;260:296–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

