In vivo characterization of target cells for acute elephant endotheliotropic herpesvirus (EEHV) infection in Asian elephants (Elephas maximus)
- PMID: 32647124
- PMCID: PMC7347588
- DOI: 10.1038/s41598-020-68413-4
In vivo characterization of target cells for acute elephant endotheliotropic herpesvirus (EEHV) infection in Asian elephants (Elephas maximus)
Abstract
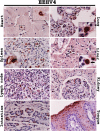
Elephant endotheliotropic herpesvirus-hemorrhagic disease (EEHV-HD) is a dangerous viral infectious disease in young Asian elephants. Despite hypotheses underlying pathogenesis of the disease, it is unclear which cell types the virus targets during acute or persistent infections. This study investigated the tissues and target cells permissive for EEHV infection and replication in vivo. Rabbit polyclonal antibodies against the non-structural proteins of EEHV, DNA polymerase (EEHV DNAPol), were generated and validated. These were used to examine EEHV infection and replication in various tissues of acute EEHV-HD cases and compared to an EEHV-negative control. The results indicated that viral antigens were distributed throughout the epithelia of the alimentary tract and salivary glands, endothelia and smooth muscle cells, and monocytic lineage cells of the EEHV-infected elephants. Moreover, EEHV DNAPol proteins were also found in the bone marrow cells of the EEHV1A-HD and EEHV1A/4-HD cases. This study demonstrated for the first time the target cells that favor in vivo EEHV replication during acute infection, providing a promising foundation for investigating EEHV propagation in vitro.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Dastjerdi A, Seilern-Moy K, Darpel K, Steinbach F, Molenaar F. Surviving and fatal Elephant Endotheliotropic Herpesvirus-1A infections in juvenile Asian elephants—Lessons learned and recommendations on anti-herpesviral therapy. BMC Vet. Res. 2016;12:178. doi: 10.1186/s12917-016-0806-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

