Tensile force-induced PDGF-BB/PDGFRβ signals in periodontal ligament fibroblasts activate JAK2/STAT3 for orthodontic tooth movement
- PMID: 32647179
- PMCID: PMC7347599
- DOI: 10.1038/s41598-020-68068-1
Tensile force-induced PDGF-BB/PDGFRβ signals in periodontal ligament fibroblasts activate JAK2/STAT3 for orthodontic tooth movement
Abstract
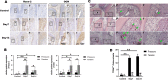
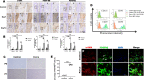
Orthodontic force-induced osteogenic differentiation and bone formation at tension side play a pivotal role in orthodontic tooth movement (OTM). Platelet-derived growth factor-BB (PDGF-BB) is a clinically proven growth factor during bone regeneration process with unclear mechanisms. Fibroblasts in periodontal ligament (PDL) are considered to be mechanosensitive under orthodontic force. Thus, we established OTM model to investigate the correlation between PDGF-BB and fibroblasts during bone regeneration at tension side. We confirmed that tensile force stimulated PDL cells to induce osteogenic differentiation via Runx-2, OCN up-regulation, and to accelerate new bone deposition along the periodontium and the alveolar bone interface. Interestingly, PDGF-BB level was remarkably enhanced at tension side during OTM in parallel with up-regulated PDGFRβ+/α-SMA+ fibroblasts in PDL by immunohistochemistry. Moreover, orthodontic force-treated primary fibroblasts from PDL were isolated and, cultured in vitro, which showed similar morphology and phenotype with control fibroblasts without OTM treatment. PDGFRβ expression was confirmed to be increased in orthodontic force-treated fibroblasts by immunofluorescence and flow cytometry. Bioinformatics analysis identified that PDGF-BB/PDGFRβ signals were relevant to the activation of JAK/STAT3 signals. The protein expression of JAK2 and STAT3 was elevated in PDL of tension side. Importantly, in vivo, the treatment of the inhibitors (imatinib and AG490) for PDGFRβ and JAK-STAT signals were capable of attenuating the tooth movement. The osteogenic differentiation and bone regeneration in tension side were down-regulated upon the treatment of inhibitors during OTM. Meanwhile, the expressions of PDGFRβ, JAK2 and STAT3 were inhibited by imatinib and AG490. Thus, we concluded that tensile force-induced PDGF-BB activated JAK2/STAT3 signals in PDGFRβ+ fibroblasts in bone formation during OTM.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. 1989;239:263–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

