Impaired iloprost-induced platelet inhibition and phosphoproteome changes in patients with confirmed pseudohypoparathyroidism type Ia, linked to genetic mutations in GNAS
- PMID: 32647264
- PMCID: PMC7347634
- DOI: 10.1038/s41598-020-68379-3
Impaired iloprost-induced platelet inhibition and phosphoproteome changes in patients with confirmed pseudohypoparathyroidism type Ia, linked to genetic mutations in GNAS
Abstract
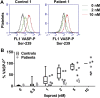
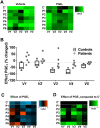
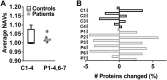
Patients diagnosed with pseudohypoparathyroidism type Ia (PHP Ia) suffer from hormonal resistance and abnormal postural features, in a condition classified as Albright hereditary osteodystrophy (AHO) syndrome. This syndrome is linked to a maternally inherited mutation in the GNAS complex locus, encoding for the GTPase subunit Gsα. Here, we investigated how platelet phenotype and omics analysis can assist in the often difficult diagnosis. By coupling to the IP receptor, Gsα induces platelet inhibition via adenylyl cyclase and cAMP-dependent protein kinase A (PKA). In platelets from seven patients with suspected AHO, one of the largest cohorts examined, we studied the PKA-induced phenotypic changes. Five patients with a confirmed GNAS mutation, displayed impairments in Gsα-dependent VASP phosphorylation, aggregation, and microfluidic thrombus formation. Analysis of the platelet phosphoproteome revealed 2,516 phosphorylation sites, of which 453 were regulated by Gsα-PKA. Common changes in the patients were: (1) a joint panel of upregulated and downregulated phosphopeptides; (2) overall PKA dependency of the upregulated phosphopeptides; (3) links to key platelet function pathways. In one patient with GNAS mutation, diagnosed as non-AHO, the changes in platelet phosphoproteome were reversed. This combined approach thus revealed multiple phenotypic and molecular biomarkers to assist in the diagnosis of suspected PHP Ia.
Conflict of interest statement
JWMH is co-founder and shareholder of FlowChamber. Other authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

