Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis
- PMID: 32647323
- PMCID: PMC7787977
- DOI: 10.1038/s41375-020-0954-2
Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis
Abstract
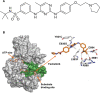
Myeloproliferative neoplasm (MPN)-associated myelofibrosis (MF) is characterized by cytopenias, marrow fibrosis, constitutional symptoms, extramedullary hematopoiesis, splenomegaly, and shortened survival. Constitutive activation of the janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway in MF leads to cell proliferation, inhibition of cell death, and clonal expansion of myeloproliferative malignant cells. Fedratinib is a selective oral JAK2 inhibitor recently approved in the United States for treatment of adult patients with intermediate-2 or high-risk MF. In mouse models of JAK2V617F-driven myeloproliferative disease, fedratinib blocked phosphorylation of STAT5, increased survival, and improved MF-associated disease features, including reduction of white blood cell counts, hematocrit, splenomegaly, and fibrosis. Fedratinib exerts off-target inhibitory activity against bromodomain-containing protein 4 (BRD4); combination JAK/STAT and BRD4 inhibition was shown to synergistically block NF-kB hyperactivation and inflammatory cytokine production, attenuating disease burden and reversing bone marrow fibrosis in animal models of MPNs. In patients, fedratinib is rapidly absorbed and dosed once daily (effective half-life 41 h). Fedratinib showed robust clinical activity in JAK-inhibitor-naïve patients and in patients with MF who were relapsed, refractory, or intolerant to prior ruxolitinib therapy. Fedratinib is effective regardless of JAK2 mutation status. Onset of spleen and symptom responses are typically seen within the first 1-2 months of treatment. The most common adverse events (AEs) with fedratinib are grades 1-2 gastrointestinal events, which are most frequent during early treatment and decrease over time. Treatment discontinuation due to hematologic AEs in clinical trials was uncommon (~3%). Suspected cases of Wernicke's encephalopathy were reported during fedratinib trials in ~1% of patients; thiamine levels should be monitored before and during fedratinib treatment as medically indicated. Phase III trials are ongoing to assess fedratinib effects on long-term safety, efficacy, and overall survival. The recent approval of fedratinib provides a much-needed addition to the limited therapeutic options available for patients with MF.
Conflict of interest statement
MT consulted for BMS and Celgene; received research funding from NS Pharma, Incyte, Stemline, Janssen, Promedior, Gilead, CTI Biopharma, Novartis, Samus Therapeutics, Asana, and Constellation; and received funding for travel and accommodations expenses from BMS and Celgene. J-JK consulted for Novartis, Roche, Celgene, and AOP Orphan; received research funding from Novartis; and received funding for travel and accommodations expenses from Celgene.
Figures


References
-
- Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1262–71. - PubMed
-
- Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7. - PubMed
-
- Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

