Experimental and theoretical study on structure-tautomerism among edaravone, isoxazolone, and their heterocycles derivatives as antioxidants
- PMID: 32647483
- PMCID: PMC7335820
- DOI: 10.1016/j.jsps.2020.06.001
Experimental and theoretical study on structure-tautomerism among edaravone, isoxazolone, and their heterocycles derivatives as antioxidants
Abstract
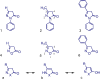
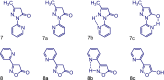
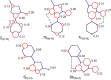
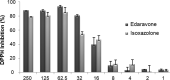
Edaravone is a heterocyclic pyrazolone compound. It has pronounced effect against free radicals, however renal and hepatic disorders have been reported. Isoxazolones are considered bioisosteric analogues of pyrazolones and may have comparable properties. Thus, we investigated the structural and electronic influences for edaravone, isoxazolone, and their tautomers on antioxidant process. Structure and tautomerism study among edaravone, isoxazolone and their heterocycles derivatives were related to antioxidant mechanisms by using the hybrid DFT method B3LYP with the basis sets 6-31++G(2d,2p). The C-H tautomer was the most stable and energetically favored among them. Intramolecular N-H-N hydrogen bonds and polar medium were responsible for the low energy differences among all possible tautomers. N-H tautomers in both systems proved to be better antioxidant by SET (single electron transfer), while O-H tautomers were better antioxidant on HAT (homolytic hydrogen atom transfer) mechanism. Theoretical calculation showed that edaravone is more potent than phenylisoxazolone, however, both has similar antioxidant scavenging on experimental DPPH. The carbonyliminic system played a very important role in the antioxidant activity for both studied classes.
Keywords: Antioxidant; DFT; DPPH; Edaravone; Heterocycles; Isoxazolone; Tautomerism.
© 2020 Published by Elsevier B.V. on behalf of King Saud University.
Figures


References
-
- Al-Salahi R., Anouar E.-H., Marzouk M., Taie H.A., Abuelizz H.A. Screening and evaluation of antioxidant activity of some 1, 2, 4-triazolo [1, 5-a] quinazoline derivatives. Future Med. Chem. 2018;10:379–390. - PubMed
-
- Alves C.N., Borges R.S., Da Silva A.B.F. Density functional theory study of metabolic derivatives of the oxidation of paracetamol. Int. J. Quantum Chem. 2006;106:2617–2623.
-
- Antonczak S. Electronic description of four flavonoids revisited by DFT method. J. Mol. Struct. 2008;856:38–45.
-
- Bandgar B.P., Gawande S.S., Bodade R.G., Gawande N.M., Khobragade C.N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009;17:8168–8173. - PubMed
LinkOut - more resources
Full Text Sources

