A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements
- PMID: 32647629
- PMCID: PMC7325125
- DOI: 10.1159/000507370
A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements
Abstract
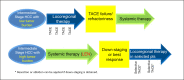
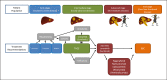
The Asia-Pacific Primary Liver Cancer Expert (APPLE) Consensus Statement on the treatment strategy for patients with intermediate-stage hepatocellular carcinoma (HCC) was established on August 31, 2019, in Sapporo, Hokkaido during the 10th Annual APPLE Meeting. This manuscript summarizes the international consensus statements developed at APPLE 2019. Transarterial chemoembolization (TACE) is the only guideline-recommended global standard of care for intermediate-stage HCC. However, not all patients benefit from TACE because intermediate-stage HCC is a heterogeneous disease in terms of tumor burden and liver function. Ten important clinical questions regarding this stage of HCC were raised, and consensus statements were generated based on high-quality evidence. In intermediate-stage HCC, preservation of liver function is as important as achieving a high objective response (OR) because the treatment goal is to prolong overall survival. Superselective conventional TACE (cTACE) is recommended as the first choice of treatment in patients eligible for effective (curative) TACE, whereas in patients who are not eligible, systemic therapy is recommended as the first choice of treatment. TACE is not indicated as the first-line therapy in TACE-unsuitable patients. Another important statement is that TACE should not be continued in patients who develop TACE failure/refractoriness in order to preserve liver function. Targeted therapy is the recommended first-line treatment for TACE-unsuitable patients. Especially, the drug, which can have higher OR rate, is preferred. Immunotherapy, transarterial radioembolization, TACE + targeted therapy or other modalities may be considered alternative options in TACE-unsuitable patients who are not candidates for targeted therapy. Better liver function, such as albumin-bilirubin grade 1, is an important factor for maximizing the therapeutic effect of systemic therapy.
Keywords: Asia-Pacific Primary Liver Cancer Expert; Hepatocellular carcinoma; Intermediate stage; Systemic therapy; Transarterial chemoembolization.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
M.K. received lecture fees from Bayer, Eisai, MSD, and Bristol-Myers Squibb, research grants from Chugai, Otsuka, Takeda, Taiho, Daiichi Sankyo, Eisai, AbbVie, EA Pharma, Gilead Sciences, Astellas Pharma, and Bristol-Myers Squibb, and advisory consulting fees from MSD, Eisai, Bayer, Bristol-Myers Squibb, Roche, and Ono Pharmaceutical. K.-H.H. received lecture fees from Eisai, Gilead Sciences and AstraZeneca and research grants from Bristol-Myers Squibb, Eisai and Ono Pharmaceutical. S.-L.Y., Z.J., Y.-H.H., and S.-M.L. has no conflicts of interest to declare. C.-K.W. had no conflict of interest. M.I. received honoraria from Eisai, Bayer, and Lilly and received research funds from Lilly, Bayer, Takeda, Eisai, Bristol Myers Squibb, AstraZeneca, Novartis, Chugai, and Merck biopharma. S.L.C. received advisory consulting fees from Eisai, MSD, Ipsen, and AstraZeneca, and research fund from Eisai, Bayer, and MSD. S.P.C. received honoraria and consulting fees from Eisai, Bayer, BMS, Roche, Celgene and AstraZeneca. Also received speaker fees from BMS, Eisai and Lilly. S.M. received honoraria (speaker fees) from Eisai, Guerbet, Bayer, MSD, Bristol-Myers, Daiichi Sankyo, Asahi Intecc, Terumo, Philips, and Piolax. A.L.C. received consultant from Bristol-Myers Squibb, Bayer Schering Pharma, Novartis, Eisai, Ono Pharmaceutical, AstraZeneca, Genentech/Roche, CSR Pharma Group, Inc., MSD, BeiGene, Ltd., Bayer Yakuhin, ISPEN, and Eli Lilly.
Figures


References
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012 Mar;379((9822)):1245–55. - PubMed
-
- Piscaglia F, Bolondi L. The intermediate hepatocellular carcinoma stage: Should treatment be expanded? Dig Liver Dis. 2010 Jul;42(Suppl 3):S258–63. - PubMed
-
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016 Apr;150((4)):835–53. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul;359((4)):378–90. - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar;391((10126)):1163–73. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

