Feasibility of Tablet-Based Patient-Reported Symptom Data Collection Among Hemodialysis Patients
- PMID: 32647760
- PMCID: PMC7335968
- DOI: 10.1016/j.ekir.2020.04.021
Feasibility of Tablet-Based Patient-Reported Symptom Data Collection Among Hemodialysis Patients
Abstract
Introduction: Individuals receiving in-center hemodialysis have high symptom burdens but often do not report their symptoms to care teams. Evidence from other diseases suggest that use of symptom electronic patient-reported outcome measures (ePROMs) may improve outcomes. We assessed the usability of a symptom ePROM system and then implemented a quality improvement (QI) project with the objective of improving symptom communication at a US hemodialysis clinic. During the project, we assessed the feasibility of ePROM implementation and conducted a substudy exploring the effect of ePROM use on patient-centered care.
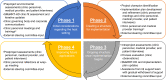
Methods: After conducting usability testing, we used mixed methods, guided by the Quality Implementation Framework, to implement a 16-week symptom ePROM QI project. We performed pre-, intra-, and postproject stakeholder interviews to identify implementation barriers and facilitators. We collected ePROM system-generated data on symptoms, e-mail alerts, and response rates, among other factors, to inform our feasibility assessment. We compared pre- and postproject outcomes.
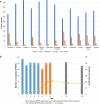
Results: There were 62 patient participants (34% black, 16% Spanish-speaking) and 19 care team participants (4 physicians, 15 clinic personnel) at QI project start, and 32 research participants. In total, the symptom ePROM was administered 496 times (completion rate = 84%). The implementation approach and ePROM system were modified to address stakeholder-identified concerns throughout. ePROM implementation was feasible as demonstrated by the program's acceptability, demand, implementation success, practicality, integration in care, and observed trend toward improved outcomes.
Conclusions: Symptom ePROM administration during hemodialysis is feasible. Trials investigating the effectiveness of symptom ePROMs and optimal administration strategies are needed.
Keywords: hemodialysis; implementation; improvement; mixed methods; patient-reported outcomes; quality; symptoms.
© 2020 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
-
- Weisbord S.D., Fried L.F., Mor M.K. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2:960–967. - PubMed
LinkOut - more resources
Full Text Sources

