Results of an Early Access Treatment Protocol of Daratumumab Monotherapy in Spanish Patients With Relapsed or Refractory Multiple Myeloma
- PMID: 32647799
- PMCID: PMC7306316
- DOI: 10.1097/HS9.0000000000000380
Results of an Early Access Treatment Protocol of Daratumumab Monotherapy in Spanish Patients With Relapsed or Refractory Multiple Myeloma
Abstract
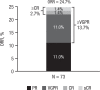
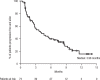
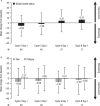
Daratumumab is a human CD38-targeted monoclonal antibody approved as monotherapy for heavily pretreated relapsed and refractory multiple myeloma. We report findings for the Spanish cohort of an open-label treatment protocol that provided early access to daratumumab monotherapy and collected safety and patient-reported outcomes data for patients with relapsed or refractory multiple myeloma. At 15 centers across Spain, intravenous daratumumab (16 mg/kg) was administered to 73 patients who had ≥3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory drug, or who were double refractory to both. The median duration of daratumumab treatment was 3.3 (range: 0.03-13.17) months, with a median number of 12 (range: 1-25) infusions. Grade 3/4 treatment-emergent adverse events were reported in 74% of patients and included lymphopenia (28.8%), thrombocytopenia (27.4%), neutropenia (21.9%), leukopenia (19.2%), and anemia (15.1%). Common (>5%) serious treatment-emergent adverse events included respiratory tract infection (9.6%), general physical health deterioration (6.8%), and back pain (5.5%). Infusion-related reactions occurred in 45% of patients. The median change from baseline in all domains of the EQ-5D-5L and EORTC QLQ-C30 was mostly 0. A total of 18 (24.7%) patients achieved a partial response or better, with 10 (13.7%) patients achieving a very good partial response or better. Median progression-free survival was 3.98 months. The results of this early access treatment protocol are consistent with previously reported trials of daratumumab monotherapy and confirm its safety and antitumoral efficacy in Spanish patients with heavily treated relapsed or refractory multiple myeloma. European Clinical Trials Database number: 2015-002993-19.
Copyright © 2020 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
